Oxidation State Of Sulfur In H2so4
Kalali
Mar 22, 2025 · 6 min read
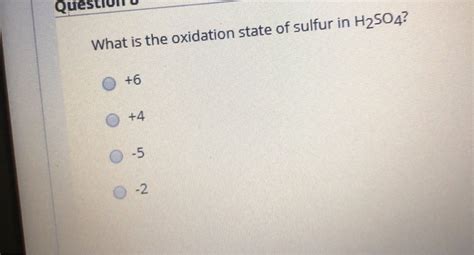
Table of Contents
Determining the Oxidation State of Sulfur in H₂SO₄
Sulfuric acid (H₂SO₄), a cornerstone chemical in numerous industrial processes, presents a fascinating case study in oxidation states. Understanding the oxidation state of sulfur within this crucial molecule is fundamental to grasping its reactivity and diverse applications. This comprehensive article will delve into the methods of determining the oxidation state of sulfur in H₂SO₄, exploring its implications and significance in chemistry.
Understanding Oxidation States
Before we pinpoint the oxidation state of sulfur in sulfuric acid, let's establish a firm understanding of the concept itself. The oxidation state, also known as the oxidation number, is a number assigned to an atom in a chemical compound that represents the hypothetical charge that atom would have if all bonds to atoms of different elements were 100% ionic. It's a crucial tool for predicting the reactivity of elements and balancing redox reactions.
Several rules govern the assignment of oxidation states:
- Free elements: The oxidation state of an atom in its elemental form is always 0. For example, the oxidation state of sulfur in S₈ is 0.
- Monatomic ions: The oxidation state of a monatomic ion is equal to its charge. For example, the oxidation state of sodium in Na⁺ is +1.
- Fluorine: Fluorine, the most electronegative element, always has an oxidation state of -1 in its compounds.
- Oxygen: Oxygen usually has an oxidation state of -2 in its compounds, except in peroxides (where it's -1) and compounds with fluorine (where it can be positive).
- Hydrogen: Hydrogen usually has an oxidation state of +1 in its compounds, except in metal hydrides (where it's -1).
- Sum of oxidation states: The sum of the oxidation states of all atoms in a neutral compound is 0, while in a polyatomic ion, it equals the charge of the ion.
Determining the Oxidation State of Sulfur in H₂SO₄
Now, let's apply these rules to determine the oxidation state of sulfur in H₂SO₄. We'll use a systematic approach:
-
Identify the known oxidation states: We know that hydrogen typically has an oxidation state of +1 and oxygen usually has an oxidation state of -2.
-
Assign the known oxidation states: In H₂SO₄, we have two hydrogen atoms (+1 each) and four oxygen atoms (-2 each).
-
Set up an equation: Let 'x' represent the oxidation state of sulfur. The sum of the oxidation states of all atoms in the neutral H₂SO₄ molecule must equal zero. Therefore, we can write the equation:
2(+1) + x + 4(-2) = 0
-
Solve for x: Simplifying the equation, we get:
2 + x - 8 = 0 x - 6 = 0 x = +6
Therefore, the oxidation state of sulfur in H₂SO₄ is +6.
Implications of the +6 Oxidation State
The +6 oxidation state of sulfur in H₂SO₄ has significant implications:
-
Strong Oxidizing Agent: Sulfur in its +6 oxidation state is relatively high. This means it has a strong tendency to accept electrons and be reduced to a lower oxidation state. Consequently, concentrated sulfuric acid acts as a strong oxidizing agent under certain conditions, particularly at high temperatures. This property is exploited in many chemical reactions.
-
Acid Strength: The high oxidation state of sulfur contributes to the exceptional strength of sulfuric acid. The highly electronegative oxygen atoms draw electron density away from the sulfur atom, weakening the S-O-H bonds and making the release of protons (H⁺) much easier. This leads to its complete dissociation in aqueous solutions.
-
Reactivity: The +6 oxidation state influences the reactivity of sulfuric acid with various substances. For example, it can react with metals to produce sulfates, often accompanied by the reduction of sulfur to a lower oxidation state (e.g., SO₂).
-
Formation of Sulfates: The high oxidation state of sulfur allows it to readily form stable sulfate salts (SO₄²⁻) with a wide range of metals. These salts are ubiquitous in nature and have diverse applications in various industries.
Comparison with Other Sulfur Compounds
To further illustrate the significance of the +6 oxidation state, let's compare it with other sulfur compounds:
-
Hydrogen Sulfide (H₂S): In H₂S, sulfur has an oxidation state of -2. This is the lowest common oxidation state for sulfur, and H₂S is a reducing agent.
-
Sulfur Dioxide (SO₂): Sulfur in SO₂ has an oxidation state of +4. SO₂ is a somewhat weaker oxidizing agent than H₂SO₄ and can also act as a reducing agent depending on the reaction conditions.
-
Sulfur Trioxide (SO₃): Sulfur in SO₃ has an oxidation state of +6, just like in H₂SO₄. SO₃ is an acidic anhydride that readily reacts with water to form sulfuric acid.
Applications of Sulfuric Acid
The unique properties of sulfuric acid, stemming from the +6 oxidation state of sulfur, underlie its extensive use across numerous industries:
-
Fertilizers: Sulfuric acid is a vital component in the production of phosphate fertilizers, which are essential for agriculture.
-
Petroleum Refining: It's used in the refining of petroleum products to remove impurities and improve their quality.
-
Metal Processing: It is used in the processing of metals such as copper, zinc, and nickel. It's also used in pickling, a process to remove oxides from metal surfaces.
-
Chemical Manufacturing: Sulfuric acid serves as a crucial reactant and catalyst in the production of a vast array of chemicals, including dyes, plastics, and detergents.
-
Battery Production: It's an electrolyte in lead-acid batteries, powering vehicles and other applications.
Conclusion
The determination of the oxidation state of sulfur in H₂SO₄ as +6 is not merely an academic exercise. This number provides profound insight into the molecule's chemical behavior, its remarkable properties, and its widespread industrial applications. Understanding this oxidation state is paramount for comprehending the reactivity of sulfuric acid and its indispensable role in numerous chemical processes. The strong oxidizing capability, exceptional acid strength, and ability to form stable sulfates all stem directly from this key characteristic. From fertilizers to batteries, the influence of the +6 oxidation state of sulfur in H₂SO₄ is far-reaching and undeniable, solidifying its position as one of the most important chemicals in the world.
Further Exploration
While this article provides a comprehensive understanding of the oxidation state of sulfur in sulfuric acid, further exploration can involve investigating:
- Redox reactions involving sulfuric acid: Investigate specific reactions where sulfuric acid acts as an oxidizing or reducing agent, paying close attention to the changes in oxidation states.
- The structure of the sulfuric acid molecule: Delve deeper into the molecular geometry and bonding within H₂SO₄, relating it to the sulfur's oxidation state.
- The industrial production of sulfuric acid: Explore the Contact process, the primary method for sulfuric acid production, emphasizing the role of sulfur oxidation states during various stages.
- Environmental impact of sulfuric acid: Investigate the environmental implications of sulfuric acid production and its uses, particularly its role in acid rain formation.
By exploring these areas, you can gain a more profound and nuanced understanding of this crucial chemical compound and its role in the world around us.
Latest Posts
Latest Posts
-
As A Sample Of Matter Is Heated Its Particles
May 09, 2025
-
Differences Between Meiosis 1 And 2
May 09, 2025
-
How Far Is Jupiter From The Sun In Au
May 09, 2025
-
How Much Is A 1 2
May 09, 2025
-
How Many Cups In 32 Ounces Of Liquid
May 09, 2025
Related Post
Thank you for visiting our website which covers about Oxidation State Of Sulfur In H2so4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.