Packages Proteins For Transport Out Of The Cell
Kalali
Mar 25, 2025 · 5 min read
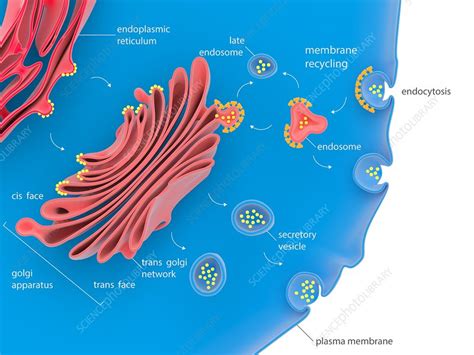
Table of Contents
Packaging Proteins for Transport Out of the Cell: A Comprehensive Guide
The intricate process of protein secretion, the carefully orchestrated movement of proteins from their site of synthesis to their final destination outside the cell, is fundamental to the survival and function of all eukaryotic cells. This journey involves a complex series of steps, each meticulously controlled to ensure efficient and accurate delivery. This article delves deep into the fascinating world of protein packaging and transport, examining the key players, mechanisms, and potential implications of dysfunction.
The Secretory Pathway: A Cellular Highway System
The secretory pathway is the cellular infrastructure responsible for transporting proteins destined for secretion, the cell membrane, or other organelles. This pathway encompasses several key compartments:
1. Ribosomes: The Protein Factories
Protein synthesis begins on ribosomes, either free in the cytoplasm or bound to the endoplasmic reticulum (ER). Proteins destined for secretion possess a specific signal sequence, a short stretch of amino acids at their N-terminus, that acts as a zip code, directing them to the ER.
2. Endoplasmic Reticulum (ER): The First Sorting Station
The ER, a vast network of interconnected membranes, is the initial processing and quality control center for secretory proteins. Once the signal sequence emerges from the ribosome, it binds to a signal recognition particle (SRP), halting translation temporarily. The SRP-ribosome complex then docks with an SRP receptor on the ER membrane. This initiates the translocation process, where the growing polypeptide chain is threaded into the ER lumen.
Signal Peptidase and Protein Folding:
Within the ER lumen, the signal sequence is cleaved off by signal peptidase, leaving a mature protein. Molecular chaperones within the ER, such as BiP (Binding immunoglobulin protein), assist in proper protein folding and prevent aggregation. Improperly folded proteins are recognized and targeted for degradation by the ER-associated degradation (ERAD) pathway. This quality control mechanism is crucial for maintaining cellular homeostasis and preventing the secretion of potentially harmful misfolded proteins.
3. Golgi Apparatus: The Processing and Packaging Center
After exiting the ER, proteins travel to the Golgi apparatus, a series of flattened membrane sacs called cisternae. The Golgi acts as a central processing and sorting station. Proteins undergo further modifications here, including glycosylation (addition of sugar chains), phosphorylation, and proteolytic cleavage. These modifications can affect protein stability, function, and targeting.
Golgi Cisternae and Compartmentalization:
The Golgi is organized into distinct compartments, each with specific enzymes responsible for particular modifications. Proteins move through the Golgi cisternae in a directional manner, undergoing a series of modifications as they progress. This compartmentalization ensures the precise and sequential processing of proteins.
4. Vesicle Transport: The Delivery System
The final stage of the secretory pathway involves the packaging of proteins into transport vesicles. These vesicles bud from the trans-Golgi network (TGN), the exit face of the Golgi. The specific destination of a protein depends on the presence of specific targeting signals within its amino acid sequence or attached carbohydrate chains.
Coat Proteins and Vesicle Formation:
The formation of transport vesicles involves coat proteins, such as COPI, COPII, and clathrin. These proteins shape the vesicle membrane, select cargo proteins for inclusion, and facilitate vesicle budding. Different coat proteins are associated with different transport steps within the secretory pathway.
5. Targeting and Fusion: Reaching the Destination
Transport vesicles move along microtubules, guided by motor proteins, to their target destinations. The vesicle membrane fuses with the target membrane, releasing the protein cargo into its final location. This fusion process requires specific recognition and interaction between vesicle and target membrane proteins.
Key Players in Protein Packaging and Transport
Several key proteins and molecules play critical roles in the intricate choreography of protein secretion:
- Signal Recognition Particle (SRP): Recognizes and binds to signal sequences, targeting ribosomes to the ER.
- Signal Peptidase: Cleaves the signal sequence from the nascent polypeptide chain in the ER lumen.
- Molecular Chaperones (e.g., BiP): Assist in protein folding and prevent aggregation.
- Protein Disulfide Isomerase (PDI): Catalyzes the formation and rearrangement of disulfide bonds, contributing to protein folding and stability.
- Glycosylation Enzymes: Modify proteins by adding sugar chains.
- Coat Proteins (COPI, COPII, Clathrin): Mediate vesicle formation and cargo selection.
- Rab Proteins: Regulate vesicle trafficking and fusion.
- SNARE Proteins: Facilitate membrane fusion.
- Motor Proteins (e.g., Kinesin, Dynein): Transport vesicles along microtubules.
Quality Control Mechanisms: Ensuring Protein Integrity
The secretory pathway incorporates several quality control checkpoints to ensure only properly folded and functional proteins are secreted. These mechanisms include:
- ERAD (ER-Associated Degradation): Misfolded proteins in the ER are retrotranslocated to the cytoplasm and degraded by the proteasome.
- Chaperone-mediated folding: Molecular chaperones assist in protein folding and prevent aggregation.
- Glycosylation quality control: Glycosylation patterns can signal the quality of protein folding.
Clinical Relevance: Diseases Related to Secretory Pathway Dysfunction
Dysfunction of the secretory pathway can have severe consequences, leading to various human diseases. These include:
- Cystic fibrosis: Caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, impairing chloride ion transport.
- Inherited disorders of glycosylation (CDGs): Result from defects in glycosylation enzymes, leading to a range of clinical manifestations.
- Neurodegenerative diseases: Accumulation of misfolded proteins in the brain can contribute to neurodegenerative disorders, such as Alzheimer's and Parkinson's disease.
- Cancer: Altered protein secretion and trafficking contribute to cancer cell proliferation, invasion, and metastasis.
Technological Advancements in Studying Protein Secretion
Technological advancements have significantly enhanced our understanding of protein secretion. These include:
- Fluorescence microscopy: Visualizes protein trafficking in real-time.
- Proteomics: Identifies and quantifies proteins involved in the secretory pathway.
- Gene editing techniques (CRISPR-Cas9): Allows for targeted manipulation of genes involved in protein secretion.
Future Directions: Unraveling the Complexities of Protein Secretion
Despite significant progress, many aspects of protein secretion remain poorly understood. Future research will focus on:
- Unraveling the precise mechanisms of vesicle trafficking and fusion.
- Identifying novel components of the secretory pathway.
- Developing new therapeutic strategies to target secretory pathway defects.
- Understanding the role of protein secretion in health and disease.
Conclusion:
The packaging and transport of proteins out of the cell is a highly complex and tightly regulated process, essential for cellular function and organismal survival. This intricate pathway involves multiple organelles, a variety of proteins, and intricate quality control mechanisms. Further research into the mechanisms and regulation of this pathway is critical to understand its role in health and disease, potentially leading to novel therapeutic strategies for a range of human disorders. The field continues to evolve rapidly, promising exciting new discoveries in the years to come.
Latest Posts
Latest Posts
-
Barrier Protection Is Not 100 Percent Effective In Preventing Stds
Mar 25, 2025
-
4 Is What Percent Of 5
Mar 25, 2025
-
How Much Is Three Eighths Of A Cup
Mar 25, 2025
-
How Many Feet Is 24 Meters
Mar 25, 2025
-
How Many Feet Are 52 Inches
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Packages Proteins For Transport Out Of The Cell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
