Periodic Table With Valence Electrons Labeled
Kalali
Mar 19, 2025 · 7 min read
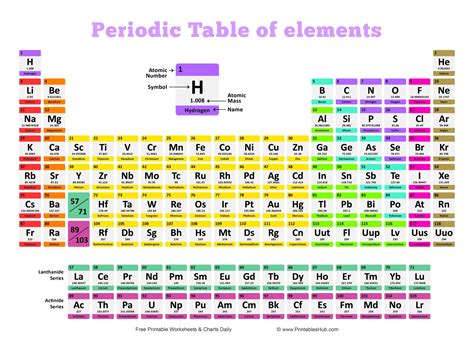
Table of Contents
The Periodic Table with Valence Electrons Labeled: A Comprehensive Guide
The periodic table is a cornerstone of chemistry, organizing elements based on their atomic structure and properties. Understanding the arrangement of elements, particularly focusing on valence electrons, is crucial for comprehending chemical bonding, reactivity, and the behavior of matter. This comprehensive guide delves into the periodic table, highlighting the importance of valence electrons and providing a detailed explanation of their role in determining chemical properties.
What is the Periodic Table?
The periodic table is a tabular arrangement of chemical elements, organized on the basis of their atomic number (number of protons), electron configuration, and recurring chemical properties. Elements are arranged in periods (rows) and groups (columns). Elements within the same group share similar chemical properties because they have the same number of valence electrons.
Understanding Valence Electrons
Valence electrons are the electrons located in the outermost shell (valence shell) of an atom. These electrons are the most loosely bound and, therefore, are the ones involved in chemical bonding. The number of valence electrons an atom possesses dictates its reactivity and the types of bonds it can form. For example, elements with one or two valence electrons tend to lose these electrons easily, forming positive ions (cations), while elements with six or seven valence electrons tend to gain electrons, forming negative ions (anions).
Importance of Valence Electrons in Chemical Bonding
The significance of valence electrons lies in their direct role in chemical bonding. Chemical bonds are forces that hold atoms together in molecules and compounds. These bonds arise from the interaction of valence electrons. There are several types of chemical bonds, including:
-
Ionic bonds: These bonds occur when one atom loses electrons to another atom, creating oppositely charged ions that attract each other. This transfer typically occurs between atoms with significantly different electronegativities – a measure of an atom's ability to attract electrons. Metals (with few valence electrons) readily lose electrons to nonmetals (with many valence electrons). Example: NaCl (sodium chloride), where sodium (Na) loses one electron to chlorine (Cl).
-
Covalent bonds: These bonds occur when atoms share valence electrons to achieve a more stable electron configuration. This sharing of electrons creates a strong attractive force between the atoms. Covalent bonds are common among nonmetals. Example: H₂ (hydrogen gas), where two hydrogen atoms share their single valence electron.
-
Metallic bonds: These bonds occur in metals, where valence electrons are delocalized, meaning they are not associated with any specific atom but rather move freely throughout the metal lattice. This sea of delocalized electrons accounts for the characteristic properties of metals, such as high electrical and thermal conductivity, and malleability.
The Periodic Table and Valence Electrons: A Closer Look
The periodic table is structured in a way that makes predicting the number of valence electrons relatively straightforward. The group number (vertical column) provides a clue.
Main Group Elements (Representative Elements)
For main group elements (Groups 1-2 and 13-18), the group number usually indicates the number of valence electrons. However, there are some exceptions:
- Group 1 (Alkali Metals): 1 valence electron
- Group 2 (Alkaline Earth Metals): 2 valence electrons
- Group 13 (Boron Group): 3 valence electrons
- Group 14 (Carbon Group): 4 valence electrons
- Group 15 (Pnictogens): 5 valence electrons
- Group 16 (Chalcogens): 6 valence electrons
- Group 17 (Halogens): 7 valence electrons
- Group 18 (Noble Gases): 8 valence electrons (except helium, which has 2)
Transition Metals
Transition metals (Groups 3-12) exhibit more complex behavior. They can have multiple oxidation states, meaning they can lose varying numbers of electrons when forming compounds. Predicting the exact number of valence electrons for transition metals is less straightforward than for main group elements. Their d electrons also participate in bonding, adding complexity.
Lanthanides and Actinides
The lanthanides and actinides (f-block elements) have even more intricate electron configurations, making it more challenging to determine their valence electron numbers. Their chemical properties are largely determined by the f electrons, rather than solely by the valence electrons.
Visualizing Valence Electrons on the Periodic Table
Imagine a periodic table with each element's symbol displaying its valence electron count. For instance, Hydrogen (H) would show '1', Oxygen (O) would show '6', and Neon (Ne) would show '8'. This visual representation enhances understanding of chemical reactivity and bonding. While this isn't typically seen in standard periodic tables, it's a useful mental model for understanding chemical behavior.
Predicting Chemical Behavior Using Valence Electrons
Understanding valence electrons allows for prediction of chemical behavior:
-
Reactivity: Elements with nearly full or empty valence shells are highly reactive. For example, alkali metals (Group 1) readily lose their single valence electron to achieve a stable octet (eight electrons in their outermost shell), making them highly reactive. Halogens (Group 17), with seven valence electrons, readily gain one electron to achieve a stable octet, making them reactive as well.
-
Bonding Type: The number of valence electrons determines the type of bonds an element can form. Elements with few valence electrons tend to form ionic bonds by losing electrons, while elements with many valence electrons tend to form covalent bonds by sharing electrons.
-
Oxidation States: The number of valence electrons dictates the possible oxidation states an element can exhibit. The oxidation state reflects the number of electrons an atom has gained or lost in a compound.
The Octet Rule and Valence Electrons
The octet rule is a guideline that states atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight electrons in their outermost shell. This rule is particularly relevant to main group elements. Noble gases, with their complete octet (except helium), are generally unreactive because they already possess stable electron configurations. However, the octet rule is not absolute; some elements can have stable configurations with fewer than eight valence electrons (like boron in some compounds).
Beyond the Basic Octet Rule: Expanded Octet and Incomplete Octet
The octet rule, while a helpful generalization, has limitations. Some elements can expand their octet by using empty d orbitals to accommodate more than eight valence electrons in certain compounds. This is common among elements in the third period and beyond. Conversely, some elements may have an incomplete octet, having fewer than eight valence electrons in their stable configuration.
Applications of Understanding Valence Electrons
The concept of valence electrons has numerous applications, including:
-
Predicting the formulas of compounds: Knowing the valence electrons of constituent elements enables prediction of the chemical formula of the resulting compound. For example, knowing that sodium (Na) has one valence electron and chlorine (Cl) has seven valence electrons allows for predicting the formula of sodium chloride as NaCl.
-
Understanding chemical reactions: The rearrangement of valence electrons during chemical reactions is a key aspect of understanding reaction mechanisms.
-
Designing new materials: The properties of materials are intrinsically linked to the electronic structure of their constituent elements, including the number of valence electrons. Understanding this relationship is critical in materials science and engineering for designing materials with specific properties.
-
Environmental Chemistry: The reactivity of elements, driven by valence electrons, is essential for understanding environmental processes, such as pollutant behavior and remediation strategies.
-
Biochemistry: Valence electrons are critical to understanding biological molecules and their interactions. The bonding within proteins, DNA, and other biomolecules is determined by the interactions of valence electrons.
Conclusion
The periodic table with valence electrons labeled provides a powerful tool for understanding the fundamental principles of chemistry. By grasping the concept of valence electrons and their role in chemical bonding, one can predict and explain a vast array of chemical phenomena. This knowledge is essential for advancements across many scientific fields, including materials science, environmental science, and biochemistry. While intricacies exist, particularly for transition and f-block elements, the fundamental understanding of valence electrons and their connection to the periodic table remains paramount in interpreting and predicting the behavior of matter.
Latest Posts
Latest Posts
-
15 Oz Is How Many Cups
Mar 20, 2025
-
Twenty Is What Percent Of 200
Mar 20, 2025
-
How Do You Write A Polynomial In Standard Form
Mar 20, 2025
-
How Many Pints Is In A Litre
Mar 20, 2025
-
How Many Feet Is 17 Inches
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Periodic Table With Valence Electrons Labeled . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
