Polalarity Lead To Surface Area Vs
Kalali
Mar 15, 2025 · 6 min read
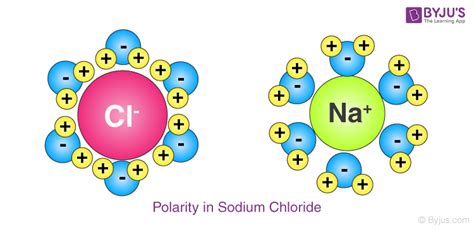
Table of Contents
Polarity's Influence on Surface Area: A Deep Dive into Molecular Interactions
Polarity plays a pivotal role in determining the surface area of a substance, particularly at the molecular level. Understanding this relationship is crucial in various fields, from materials science and chemistry to biology and environmental science. This article will delve into the intricate connection between polarity and surface area, exploring how different molecular interactions influence the overall surface area and its implications.
Understanding Polarity and its Molecular Implications
Before exploring the connection between polarity and surface area, let's establish a clear understanding of molecular polarity. Polarity arises from an uneven distribution of electron density within a molecule. This uneven distribution results from differences in electronegativity between atoms within the molecule. Electronegativity is the ability of an atom to attract electrons in a chemical bond.
Electronegativity Differences and Dipole Moments
When atoms with significantly different electronegativities bond, the more electronegative atom pulls the shared electrons closer, creating a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the less electronegative atom. This separation of charge creates a dipole moment, a vector quantity representing the magnitude and direction of the charge separation. Molecules with significant dipole moments are considered polar, while molecules with negligible or no dipole moments are considered nonpolar.
Examples of Polar and Nonpolar Molecules
Water (H₂O) is a classic example of a polar molecule. Oxygen is significantly more electronegative than hydrogen, resulting in a substantial dipole moment. In contrast, methane (CH₄) is a nonpolar molecule because the electronegativity difference between carbon and hydrogen is relatively small, leading to a nearly symmetrical distribution of electron density.
How Polarity Affects Intermolecular Forces
The polarity of a molecule dictates the type and strength of intermolecular forces it can form with other molecules. These intermolecular forces significantly impact the overall arrangement and packing of molecules, directly influencing surface area.
Hydrogen Bonding: A Strong Polar Interaction
Hydrogen bonds are a special type of dipole-dipole interaction that occurs when a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) interacts with another electronegative atom in a different molecule. Hydrogen bonds are considerably stronger than typical dipole-dipole interactions and significantly influence the properties of substances like water, which exhibits a relatively high surface tension due to extensive hydrogen bonding.
Dipole-Dipole Interactions: Moderate Polar Interactions
In polar molecules lacking hydrogen bonding capabilities, dipole-dipole interactions are the dominant intermolecular forces. These interactions involve the attraction between the positive end of one polar molecule and the negative end of another. While weaker than hydrogen bonds, dipole-dipole interactions still influence the arrangement and packing of molecules.
London Dispersion Forces: Weak Nonpolar Interactions
Even nonpolar molecules experience intermolecular forces, albeit weaker ones called London dispersion forces (LDFs). These forces arise from temporary fluctuations in electron density, creating temporary dipoles that induce dipoles in neighboring molecules. The strength of LDFs generally increases with the size and molecular weight of the molecule.
Polarity's Influence on Surface Area: Macroscopic Effects
The interplay between these intermolecular forces, driven by molecular polarity, translates into macroscopic observable effects on surface area.
Surface Tension and Polarity
Surface tension, the force that causes the surface of a liquid to contract and behave like a stretched elastic membrane, is strongly influenced by polarity. Polar molecules with strong intermolecular forces (like water) exhibit higher surface tension because the molecules at the surface are strongly attracted to each other, minimizing the surface area. Conversely, nonpolar molecules with weaker intermolecular forces have lower surface tension.
Wetting and Polarity
Wetting, the ability of a liquid to spread over a surface, is also affected by polarity. Polar liquids tend to wet polar surfaces effectively because of strong interactions between the liquid molecules and the surface molecules. Nonpolar liquids generally wet nonpolar surfaces better.
Crystal Structure and Polarity
The crystalline structure of a solid substance is directly impacted by the nature of intermolecular forces present. Polar molecules often form crystals with more compact structures due to strong intermolecular attractions, leading to a smaller overall surface area compared to the crystals formed by nonpolar molecules. The tighter packing minimizes the overall exposed surface area.
Practical Applications and Examples
Understanding the link between polarity and surface area has numerous practical applications across various scientific disciplines.
Materials Science: Designing Materials with Specific Surface Areas
In materials science, controlling the surface area of materials is crucial for numerous applications. For example, catalysts often require high surface areas to maximize their interaction with reactants. The design of porous materials, like zeolites, relies heavily on understanding how polarity influences the arrangement of molecules and hence the surface area accessible for adsorption and reactions.
Pharmaceutical Science: Drug Delivery and Absorption
The surface area of drug particles plays a significant role in their dissolution rate and absorption in the body. Modifying the polarity of drug molecules or using surfactants (surface-active agents) can alter the surface area and consequently affect the bioavailability of a drug.
Environmental Science: Pollutant Adsorption and Remediation
The adsorption of pollutants onto soil or other materials is influenced by the polarity of both the pollutants and the adsorbent materials. Polar pollutants are more likely to be adsorbed onto polar surfaces, leading to effective remediation strategies.
Advanced Considerations: Beyond Simple Polarity
While simple polarity significantly impacts surface area, other factors also play a role. These include:
- Molecular Shape and Size: The shape and size of molecules influence how efficiently they can pack together, impacting the overall surface area. Branched molecules often have larger surface areas than linear molecules with the same molecular weight.
- Temperature: Temperature affects the kinetic energy of molecules and hence the strength of intermolecular forces. Higher temperatures can reduce the effectiveness of intermolecular forces, leading to an increase in surface area.
- Pressure: Pressure can also influence the packing of molecules, affecting surface area. Higher pressures can lead to a decrease in surface area.
- Presence of Other Molecules: The presence of other molecules, such as solvents or additives, can interfere with intermolecular forces, thereby influencing surface area.
Conclusion
The relationship between polarity and surface area is complex and multifaceted, deeply rooted in the nature of intermolecular forces. Understanding this connection is critical for developing new materials, designing efficient drug delivery systems, and tackling environmental challenges. Further research into the intricate interplay of polarity, molecular interactions, and surface area continues to be crucial for advancements in various scientific fields. This deep understanding allows scientists and engineers to fine-tune material properties and create innovative solutions for a wide range of applications. The ongoing exploration of this relationship will undoubtedly lead to further breakthroughs in the years to come.
Latest Posts
Latest Posts
-
5 And 1 2 As A Decimal
Mar 15, 2025
-
How Do You Convert From Moles To Liters
Mar 15, 2025
-
What Separates A Salamander From A Turtle
Mar 15, 2025
-
Is The Atlantic Ocean Warmer Than The Pacific
Mar 15, 2025
-
Que Porcentaje Es 20 De 27
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Polalarity Lead To Surface Area Vs . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
