Reaction Of Naf To Produce Basic Solution
Kalali
Mar 28, 2025 · 6 min read
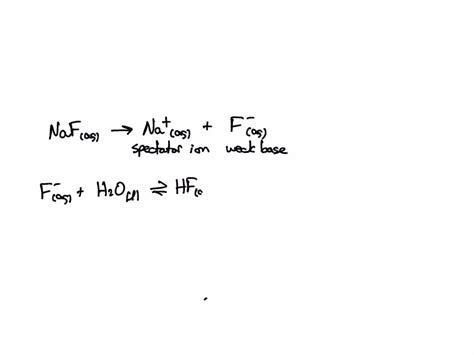
Table of Contents
The Reaction of Sodium Fluoride (NaF) to Produce a Basic Solution: A Deep Dive
Sodium fluoride (NaF), a seemingly simple ionic compound, exhibits interesting behavior when dissolved in water, leading to the formation of a slightly basic solution. This seemingly counterintuitive result stems from the hydrolysis of the fluoride ion (F⁻), a process that's crucial in various chemical applications and deserves a thorough exploration. This article will delve into the intricacies of this reaction, examining the underlying chemistry, the factors influencing the basicity of the solution, and its practical implications.
Understanding Hydrolysis: The Key to Basicity
The key to understanding why a solution of NaF is basic lies in the concept of hydrolysis. Hydrolysis is a chemical reaction where a substance reacts with water, often resulting in the breaking of chemical bonds. In the case of NaF, it's the fluoride ion (F⁻) that undergoes hydrolysis.
When NaF dissolves in water, it dissociates completely into its constituent ions: sodium ions (Na⁺) and fluoride ions (F⁻). While the sodium ion is a spectator ion, having negligible effect on the solution's pH, the fluoride ion reacts with water molecules. This reaction can be represented as follows:
F⁻(aq) + H₂O(l) ⇌ HF(aq) + OH⁻(aq)
This equilibrium shows that the fluoride ion accepts a proton (H⁺) from a water molecule, forming hydrofluoric acid (HF) and hydroxide ions (OH⁻). The presence of hydroxide ions (OH⁻) is what makes the solution basic, increasing its pH above 7.
The Role of the Equilibrium Constant (Kb)
The extent to which the hydrolysis reaction proceeds is determined by the base dissociation constant (Kb). Kb represents the equilibrium constant for the base hydrolysis reaction. A higher Kb value indicates a stronger base and a more significant increase in hydroxide ion concentration, resulting in a more basic solution.
The Kb value for the fluoride ion is relatively small, reflecting the fact that it's a weak base. This means that only a small fraction of the fluoride ions will react with water to form hydroxide ions. However, even this small increase in OH⁻ concentration is sufficient to make the solution slightly basic.
Factors Influencing the Basicity of the NaF Solution
Several factors can influence the basicity of a NaF solution, including:
1. Concentration of NaF:
The concentration of NaF directly impacts the concentration of fluoride ions (F⁻) available for hydrolysis. A higher NaF concentration leads to a higher concentration of F⁻ ions, resulting in more OH⁻ ions being produced and a more basic solution. This relationship is directly proportional: higher concentration, higher basicity.
2. Temperature:
Temperature affects the equilibrium constant (Kb). Generally, an increase in temperature increases the Kb value for most reactions. This means a higher temperature will favor the hydrolysis reaction, resulting in a slightly more basic solution. However, the effect of temperature on the basicity of a NaF solution is relatively small compared to the effect of concentration.
3. Presence of Other Ions:
The presence of other ions in the solution can affect the basicity through several mechanisms, including:
-
Common ion effect: If another source of fluoride ions is added to the solution (e.g., adding HF), the equilibrium shifts to the left, decreasing the concentration of OH⁻ and reducing the basicity.
-
Ionic strength: High ionic strength can influence the activity coefficients of the ions, thereby affecting the equilibrium position and ultimately the basicity.
-
Complexation: If other ions are capable of forming complexes with fluoride ions, this will reduce the concentration of free fluoride ions available for hydrolysis, thus decreasing the basicity.
Calculating the pH of a NaF Solution
To calculate the pH of a NaF solution, we need to consider the hydrolysis equilibrium and the Kb value for the fluoride ion. The calculation generally involves these steps:
-
Write the hydrolysis reaction: F⁻(aq) + H₂O(l) ⇌ HF(aq) + OH⁻(aq)
-
Write the Kb expression: Kb = [HF][OH⁻]/[F⁻]
-
Use an ICE table: Set up an ICE (Initial, Change, Equilibrium) table to track the changes in concentration during the reaction.
-
Solve for [OH⁻]: Substitute the equilibrium concentrations into the Kb expression and solve for the hydroxide ion concentration.
-
Calculate pOH: pOH = -log[OH⁻]
-
Calculate pH: pH = 14 - pOH
Example: Let's consider a 0.1 M solution of NaF. Assuming the Kb for F⁻ is approximately 1.5 x 10⁻¹¹, solving the equilibrium expression (using an ICE table and neglecting the x in the denominator due to the small Kb value) will give an approximate [OH⁻] concentration. This [OH⁻] can then be used to calculate the pOH and subsequently the pH, which will be slightly greater than 7, confirming the basic nature of the solution.
It's crucial to note that the precise calculation can be more complex depending on the concentration and potential presence of other ions in the solution. More sophisticated methods might be needed to account for activity coefficients.
Practical Implications of NaF's Basicity
The slightly basic nature of NaF solutions has several practical implications across various fields:
1. Dentistry:
Sodium fluoride is a crucial component in dental products like toothpaste and mouthwashes. Its slight basicity contributes to its effectiveness in preventing tooth decay by remineralizing tooth enamel. The hydroxide ions generated contribute to the neutralization of acids produced by oral bacteria.
2. Water Treatment:
NaF is added to drinking water in some regions to prevent dental caries (cavities) through fluoridation. The slight basicity of the added fluoride doesn't pose significant health risks at the concentrations used in water treatment.
3. Industrial Applications:
NaF finds applications in various industrial processes, including metal processing, pesticide production, and the manufacture of certain chemicals. Understanding its basicity is important for controlling the pH of these processes and preventing unwanted reactions.
4. Buffer Solutions:
While NaF alone doesn't create a strong buffer solution, it can be part of a buffer system in combination with its conjugate acid, hydrofluoric acid (HF). This system can resist changes in pH when small amounts of acid or base are added. Understanding the hydrolysis equilibrium is essential for designing effective buffer solutions based on the fluoride-hydrofluoric acid system.
Conclusion:
The reaction of NaF to produce a slightly basic solution is a fascinating example of the hydrolysis of a weak base. While the increase in basicity might seem subtle, it holds significant implications in various fields, from dentistry and water treatment to industrial processes and buffer solution preparation. Understanding the underlying chemistry, including the role of hydrolysis, the equilibrium constant (Kb), and influencing factors like concentration and temperature, is vital for appreciating the diverse applications and behavior of this seemingly simple ionic compound. The relatively straightforward yet nuanced nature of this reaction serves as a valuable learning opportunity in exploring equilibrium chemistry and its practical applications.
Latest Posts
Latest Posts
-
How Many Inches Are 14 Cm
Mar 31, 2025
-
How Many Centimetres In 2 Metres
Mar 31, 2025
-
How Many Meters Is 2 Kilometers
Mar 31, 2025
-
How Long Does Granular Fertilizer Last In Soil
Mar 31, 2025
-
How Many Ounces Is One Kilogram
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Reaction Of Naf To Produce Basic Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
