Region Around The Nucleus Where Electrons Are Located
Kalali
Mar 27, 2025 · 6 min read
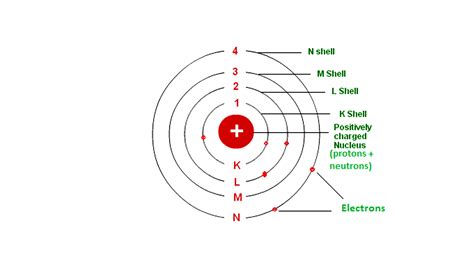
Table of Contents
The Atomic Neighborhood: Exploring the Region Around the Nucleus Where Electrons Reside
The atom, the fundamental building block of matter, is a fascinating realm of quantum mechanics. While often depicted as a miniature solar system, with electrons orbiting a central nucleus like planets around a star, the reality is far more nuanced and intriguing. This article delves deep into the region surrounding the nucleus – where electrons are found – exploring the concepts of electron shells, subshells, orbitals, and the probability distributions that govern electron behavior. We will unpack the complexities of electron configuration and its impact on an atom's chemical properties.
Understanding the Quantum Realm: Beyond Simple Orbits
The classical model of the atom, with electrons tracing neat, predictable paths, is insufficient to describe the true nature of electron behavior. Quantum mechanics dictates that electrons don't follow defined trajectories; instead, their location is described by probability. We can't pinpoint an electron's exact position at any given moment, only the probability of finding it within a particular region of space. This probabilistic nature is central to understanding the electron's environment around the nucleus.
The Electron Shell Model: Energy Levels and Distances
The simplest conceptualization is the electron shell model. Electrons occupy distinct energy levels, also known as shells, surrounding the nucleus. Shells are designated by principal quantum numbers (n), where n = 1 represents the shell closest to the nucleus (the lowest energy level), n = 2 the next, and so on. Each shell can hold a limited number of electrons, determined by the formula 2n². Thus, the first shell (n=1) can hold a maximum of 2 electrons, the second shell (n=2) can hold 8, the third (n=3) 18, and so forth.
Key takeaway: Electrons in shells farther from the nucleus possess higher energy levels. This energy difference dictates their reactivity and the atom's overall chemical behavior. Electrons in outer shells are more loosely bound and more readily participate in chemical reactions.
Delving Deeper: Subshells and Orbitals
The shell model provides a basic understanding, but it's an oversimplification. Within each shell, electrons are further organized into subshells, characterized by their angular momentum quantum number (l). For a given principal quantum number (n), the possible values of l range from 0 to n-1. These subshells are designated by letters:
- l = 0: s subshell (spherical shape)
- l = 1: p subshell (dumbbell shape)
- l = 2: d subshell (more complex shapes)
- l = 3: f subshell (even more complex shapes)
Each subshell contains one or more orbitals. An orbital is a region of space where the probability of finding an electron is high (typically 90%). The magnetic quantum number (ml) specifies the orientation of the orbital in space. For a given subshell, the number of orbitals is 2l + 1.
For instance:
- An s subshell (l=0) has one orbital.
- A p subshell (l=1) has three orbitals (px, py, pz).
- A d subshell (l=2) has five orbitals.
- An f subshell (l=3) has seven orbitals.
Visualizing Orbitals: Shapes and Probability Clouds
It's crucial to understand that orbitals aren't physical boundaries; they represent regions of high electron probability. The shapes of orbitals are often depicted graphically to illustrate the probability distribution. The s orbitals are spherical, while p orbitals have dumbbell shapes oriented along the x, y, and z axes. D and f orbitals have more complex, multi-lobed shapes. These shapes are not fixed, but rather represent the most likely location of the electron.
Imagine: Instead of thinking of electrons as tiny particles whizzing around the nucleus, visualize them as fuzzy clouds of probability, denser in some regions than others, dictated by the shape and orientation of their corresponding orbital.
Electron Configuration: Filling the Atomic Neighborhood
Electron configuration describes how electrons are distributed among the various shells, subshells, and orbitals within an atom. It follows specific rules, dictated by the principles of quantum mechanics and the Aufbau principle (building-up principle):
- Lower energy levels fill first: Electrons occupy the lowest energy levels available before filling higher energy levels.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, each with opposite spins (spin quantum number, ms = +1/2 or -1/2).
- Hund's Rule: Electrons fill orbitals within a subshell individually before pairing up. This minimizes electron-electron repulsion.
By understanding these rules, we can predict the electron configuration of any element. For example, the electron configuration of oxygen (atomic number 8) is 1s²2s²2p⁴. This indicates two electrons in the 1s orbital, two in the 2s orbital, and four in the 2p orbitals.
The Significance of Electron Configuration: Chemical Properties and Reactivity
Electron configuration is paramount in determining an atom's chemical properties and reactivity. The outermost electrons, called valence electrons, are particularly important. These electrons are involved in chemical bonding and determine how an atom interacts with other atoms. Atoms tend to react in ways that achieve a stable electron configuration, often resembling that of a noble gas (full outer shell).
Examples:
- Alkali metals (Group 1): Have one valence electron, readily lose it to form a +1 ion, exhibiting high reactivity.
- Halogens (Group 17): Have seven valence electrons, readily gain one electron to form a -1 ion, also exhibiting high reactivity.
- Noble gases (Group 18): Have a full outer shell of electrons, making them very unreactive.
Beyond the Basics: Advanced Concepts
While the shell, subshell, and orbital model provides a strong foundation, several advanced concepts further refine our understanding of electron behavior:
- Electron-electron repulsion: Electrons within the same atom repel each other, influencing orbital shapes and energies.
- Shielding effect: Inner electrons shield outer electrons from the full positive charge of the nucleus, reducing the effective nuclear charge experienced by outer electrons.
- Penetration effect: Electrons in s orbitals have a higher probability of being closer to the nucleus than electrons in other orbitals, influencing their energy levels.
- Quantum numbers and their implications: A deep understanding of the four quantum numbers (n, l, ml, ms) provides a thorough understanding of the intricacies of electron behavior and distribution.
Conclusion: A Probabilistic Landscape
The region around the nucleus where electrons reside isn't a simple, predictable arrangement. Instead, it's a dynamic, probabilistic landscape shaped by the principles of quantum mechanics. The concepts of electron shells, subshells, and orbitals provide a framework for understanding the probability distributions of electrons, offering insights into atomic structure, chemical bonding, and the properties of matter. By mastering these concepts, we can unlock a deeper appreciation of the intricate workings of the atomic world. Further exploration into advanced concepts such as molecular orbital theory and quantum field theory further expands our understanding of this fascinating area of science. The continued study of the atomic neighborhood continues to push the boundaries of our understanding of the universe around us.
Latest Posts
Latest Posts
-
How Many Liters Is 100 Ml
Mar 30, 2025
-
1 In 25 As A Percentage
Mar 30, 2025
-
For Each Action There Is A Reaction
Mar 30, 2025
-
Why Do Flowers Contain More Stamen Than Pistils
Mar 30, 2025
-
Why Is The Entropy Change Negative For Ring Closures
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Region Around The Nucleus Where Electrons Are Located . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
