Rutherford's Gold Foil Experiment Helped Prove
Kalali
Mar 31, 2025 · 6 min read
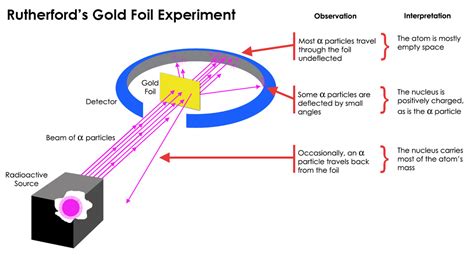
Table of Contents
Rutherford's Gold Foil Experiment: Revolutionizing Our Understanding of the Atom
Ernest Rutherford's gold foil experiment, conducted in 1909 by Hans Geiger and Ernest Marsden under Rutherford's supervision, stands as a pivotal moment in the history of physics. This seemingly simple experiment, involving alpha particles bombarding a thin gold foil, shattered the then-accepted "plum pudding" model of the atom and paved the way for our modern understanding of atomic structure. It proved several key things about the atom, fundamentally altering our perception of matter itself.
What Rutherford's Gold Foil Experiment Proved
The experiment definitively proved several crucial aspects of atomic structure:
1. The Atom is Mostly Empty Space
The most striking result of the experiment was the observation that the vast majority of alpha particles passed straight through the gold foil with minimal deflection. This indicated that the atom is not a solid, uniformly dense sphere as previously believed, but rather contains a significant amount of empty space. If the atom were a densely packed sphere of positive and negative charges as the plum pudding model suggested, the alpha particles would experience significant scattering and deflection. The fact that most passed through unimpeded directly contradicted this model.
2. The Existence of a Dense, Positively Charged Nucleus
While most alpha particles passed straight through, a small but significant number were deflected at large angles, some even bouncing directly back. This unexpected observation could only be explained by the presence of a small, dense, positively charged region within the atom that Rutherford termed the nucleus. The strong positive charge of the nucleus repelled the positively charged alpha particles, causing the significant deflections. The closer an alpha particle came to the nucleus, the greater the repulsive force and the larger the angle of deflection. This explained the few particles that underwent significant scattering.
3. The Nucleus Contains Most of the Atom's Mass
The large angle deflections, and particularly the particles that were reflected back, provided evidence for the concentration of the atom's mass within the nucleus. The immense force required to deflect the relatively massive alpha particles suggested that the nucleus contained almost all of the atom's mass. The electrons, being much less massive, contributed negligibly to the overall mass of the atom.
4. Electrons Orbit the Nucleus
The experiment, while not directly proving the existence of electron orbitals, strongly suggested the arrangement of electrons around the nucleus. Since the atom is mostly empty space and the positive charge is concentrated in the nucleus, it logically follows that the negatively charged electrons must reside outside the nucleus, in a region of empty space. While the experiment didn't define the exact nature of electron orbits (that came later with Bohr's model), it laid the foundation for understanding their arrangement.
The Plum Pudding Model and its Demise
Before Rutherford's experiment, the prevailing model of the atom was J.J. Thomson's "plum pudding" model. This model depicted the atom as a sphere of positive charge with negatively charged electrons embedded within it, much like plums in a pudding. This model, while explaining the existence of electrons, failed to account for the experimental observations of Rutherford's gold foil experiment. The plum pudding model predicted far less scattering than was actually observed, making it incompatible with the experimental results. Rutherford's experiment effectively demolished the plum pudding model, necessitating a new, more accurate model of the atom.
The Significance of Rutherford's Experiment
Rutherford's gold foil experiment is significant for several reasons:
- Revolutionized Atomic Theory: It fundamentally changed our understanding of the atom from a homogenous sphere to a complex structure with a central nucleus and orbiting electrons. This marked a paradigm shift in atomic physics.
- Foundation for Future Discoveries: The discovery of the nucleus provided the framework for further investigations into nuclear physics, leading to discoveries like isotopes, nuclear reactions, and the development of nuclear energy.
- Advanced Experimental Techniques: The experiment demonstrated the power of experimental physics in unraveling the secrets of the natural world, showcasing the importance of careful observation and interpretation of experimental data.
- Improved Atomic Models: It paved the way for the development of more sophisticated atomic models, eventually leading to the quantum mechanical model that we use today.
- Impact on Scientific Methodology: Rutherford's experiment exemplified the scientific method, showing how a carefully designed experiment can refute an established theory and lead to the creation of a better one. It highlights the crucial role of falsification in scientific progress.
Further Developments Following Rutherford's Experiment
Rutherford's experiment, while groundbreaking, left several unanswered questions. The model didn't explain the stability of the atom, as electrons orbiting the nucleus would be expected to emit radiation and spiral into the nucleus, causing the atom to collapse. This issue was addressed later by Niels Bohr's model, which introduced the concept of quantized electron orbits. Bohr’s model, while an improvement, was still incomplete and was superseded by the more accurate quantum mechanical model of the atom which fully accounts for the wave-particle duality of electrons.
The gold foil experiment also didn't explicitly define the size or structure of the nucleus. Later experiments, using techniques like electron scattering and nuclear reactions, revealed more details about the nucleus, including the existence of protons and neutrons. These further discoveries refined our understanding of the atomic structure, building upon the foundational knowledge obtained from Rutherford's seminal experiment.
The Methodology of Rutherford's Gold Foil Experiment
The experiment involved a simple yet ingenious setup. A beam of alpha particles (helium nuclei), emitted from a radioactive source (usually radon), was directed at a very thin gold foil (only a few atoms thick). A zinc sulfide screen surrounding the foil detected the scattered alpha particles. When an alpha particle struck the screen, it produced a scintillation, or a tiny flash of light, which could be observed using a microscope.
The experimenters carefully observed the pattern of scintillations on the screen. The vast majority of alpha particles passed straight through the foil, but some were deflected at various angles, with a small number even bouncing back towards the source. By meticulously recording the number of alpha particles deflected at different angles, Rutherford and his team were able to deduce the nature of the atom's internal structure.
The thinness of the gold foil was crucial. Using a thin foil ensured that the alpha particles would mostly interact with only a single atom during their passage through the foil, thus simplifying the interpretation of the scattering data. A thicker foil would have resulted in multiple scattering events, making it more difficult to analyze the results.
Conclusion: A Lasting Legacy
Rutherford's gold foil experiment stands as a testament to the power of scientific inquiry and its ability to reveal the underlying structure of matter. The experiment’s impact extends far beyond the realm of atomic physics. It serves as a powerful example of the scientific method in action, illustrating how observation, hypothesis, experimentation, and analysis can lead to a profound understanding of the natural world. Its legacy continues to inspire scientists today, emphasizing the importance of meticulous experimentation and the enduring quest to unveil the universe's deepest secrets. The experiment’s findings fundamentally shifted our understanding of the universe at a fundamental level, providing the groundwork for countless advancements in science and technology. The simple yet profound implications of Rutherford's experiment continue to shape our world in ways that were unimaginable at the time.
Latest Posts
Latest Posts
-
How Long Is 78 Inches In Feet
Apr 01, 2025
-
How Tall Is 151 Cm In Feet
Apr 01, 2025
-
How Much Fahrenheit Is 175 Celsius
Apr 01, 2025
-
Factor When A Is Not 1
Apr 01, 2025
-
Roasting A Marshmallow Physical Or Chemical Change
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Rutherford's Gold Foil Experiment Helped Prove . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
