Specific Heat Capacity Of Sand And Water
Kalali
Mar 15, 2025 · 5 min read
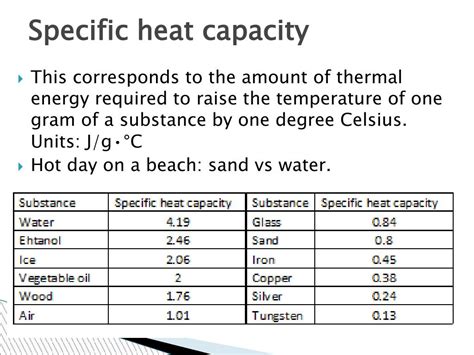
Table of Contents
Specific Heat Capacity of Sand and Water: Understanding the Differences
The seemingly simple question of why sand gets so hot on a sunny day while the ocean stays relatively cool holds a fascinating key to understanding the concept of specific heat capacity. This crucial thermodynamic property dictates how much energy is needed to raise the temperature of a substance. The stark contrast between sand and water's specific heat capacities explains not only beachside temperature differences but also has profound implications for climate, weather patterns, and even biological processes. This article will delve deep into the specific heat capacities of sand and water, exploring their differences, the underlying reasons for these differences, and the real-world consequences of these distinct properties.
What is Specific Heat Capacity?
Before diving into the specifics of sand and water, let's define specific heat capacity. Simply put, it's the amount of heat energy required to raise the temperature of one kilogram (or one gram, depending on the unit system used) of a substance by one degree Celsius (or one Kelvin). It's often denoted by the symbol 'c' and measured in Joules per kilogram-Kelvin (J/kg·K) or Joules per gram-degree Celsius (J/g·°C).
Specific heat capacity is an intensive property, meaning it doesn't depend on the amount of substance present. A small grain of sand and a massive sand dune will have the same specific heat capacity. This is unlike extensive properties like mass or volume, which are directly proportional to the amount of material.
Factors Affecting Specific Heat Capacity
Several factors influence a substance's specific heat capacity:
-
Molecular Structure: The complexity and arrangement of molecules within a substance significantly impact how efficiently they absorb and distribute energy. More complex molecules with numerous vibrational and rotational modes can store more energy, leading to a higher specific heat capacity.
-
Intermolecular Forces: Strong intermolecular forces, such as hydrogen bonds in water, require considerable energy to overcome, thus increasing the specific heat capacity. Weaker forces result in lower specific heat capacity.
-
Phase of Matter: The specific heat capacity of a substance changes depending on its phase (solid, liquid, gas). Generally, the specific heat capacity is higher in the liquid phase than in the solid phase.
Specific Heat Capacity of Sand vs. Water: A Striking Contrast
Now, let's focus on the core topic: the specific heat capacity of sand and water. Sand, primarily composed of silica (silicon dioxide), has a relatively low specific heat capacity, typically around 0.84 J/g·°C. Water, on the other hand, boasts an exceptionally high specific heat capacity of approximately 4.18 J/g·°C. This difference is approximately five times greater. This seemingly small numerical difference has vast implications.
Why the Difference? The Role of Hydrogen Bonding
The significant difference in specific heat capacity between sand and water is largely attributed to the presence of hydrogen bonds in water. Water molecules are polar, meaning they have a slightly positive end and a slightly negative end. This polarity allows water molecules to form strong hydrogen bonds with each other, creating a cohesive network. Breaking these bonds requires a substantial amount of energy, resulting in the high specific heat capacity.
Sand, being composed of mostly silica, lacks the strong intermolecular forces present in water. The weaker interactions between silica particles require significantly less energy to raise their temperature. This explains why sand heats up and cools down much faster than water.
Real-World Consequences of the Difference
The contrasting specific heat capacities of sand and water have numerous real-world consequences:
Coastal Climate Moderation
The high specific heat capacity of water plays a crucial role in moderating coastal climates. Large bodies of water, like oceans and seas, act as massive heat reservoirs. They absorb a tremendous amount of solar energy during the day without experiencing significant temperature increases. At night, they release this stored heat, preventing extreme temperature drops. This results in coastal regions experiencing milder temperatures with smaller daily and seasonal fluctuations compared to inland areas.
Marine Life and Ecosystem Stability
The high specific heat capacity of water is essential for maintaining stable marine ecosystems. The relatively constant temperature of the ocean provides a stable environment for diverse marine life, protecting them from drastic temperature changes that could disrupt their physiological processes.
Weather Patterns and Precipitation
The vast heat capacity of oceans significantly influences weather patterns and precipitation. Evaporation from the ocean's surface, fueled by absorbed solar energy, contributes to atmospheric moisture. This moisture then forms clouds and eventually leads to precipitation, playing a vital role in the global water cycle.
Industrial Applications
The specific heat capacity of both sand and water is utilized in various industrial applications. Sand's low specific heat capacity makes it suitable for applications where rapid heating and cooling are desired, such as in certain types of foundry work. Water's high specific heat capacity makes it an excellent coolant in industrial processes, power plants, and engines.
Desert Environments and Extreme Temperatures
In contrast to coastal areas, desert environments, characterized by large expanses of sand and minimal water, experience extreme temperature fluctuations. The low specific heat capacity of sand causes rapid heating during the day and rapid cooling at night, leading to significant daily temperature swings.
Further Exploring Specific Heat Capacity
Understanding specific heat capacity goes beyond simply comparing sand and water. This fundamental property has vast applications in diverse fields, including:
- Meteorology: Predicting weather patterns, understanding climate change, and analyzing atmospheric processes.
- Oceanography: Studying ocean currents, marine ecosystems, and the impact of climate change on marine environments.
- Materials Science: Designing materials with specific thermal properties for various applications.
- Engineering: Designing efficient cooling systems and managing thermal processes in various industries.
- Biology: Understanding how organisms regulate their body temperature and adapt to different environments.
Conclusion:
The difference in specific heat capacity between sand and water is not just an academic curiosity; it's a fundamental principle with far-reaching consequences. The high specific heat capacity of water moderates climate, supports marine life, and influences weather patterns. The low specific heat capacity of sand contributes to the extreme temperature fluctuations observed in desert regions. Understanding these differences is crucial for comprehending many natural phenomena and developing effective solutions for various environmental and technological challenges. Further exploration into the nuances of specific heat capacity and its impact on various systems remains an active and important area of scientific research.
Latest Posts
Latest Posts
-
What Is The Percentage Of 0 08
Mar 15, 2025
-
Which Layer Of The Earth Is More Dense
Mar 15, 2025
-
Frying An Egg Chemical Or Physical Change
Mar 15, 2025
-
What Is The Percentage Of 2 9
Mar 15, 2025
-
What Is 40 Off Of 40
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Specific Heat Capacity Of Sand And Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
