Structurally Atp Is Most Like Which Type Of Molecule
Kalali
Mar 24, 2025 · 5 min read
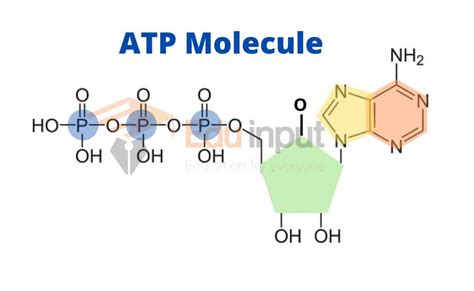
Table of Contents
Structurally, ATP is Most Like Which Type of Molecule?
ATP, or adenosine triphosphate, is the primary energy currency of all living cells. Understanding its structure is crucial to understanding its function. But structurally, what molecule does ATP most resemble? The answer isn't immediately obvious, as ATP possesses characteristics of several different molecular classes. However, a detailed comparison reveals a striking structural similarity to a specific type of molecule: nucleotides.
Understanding the Structure of ATP
Before diving into comparisons, let's establish a firm understanding of ATP's structure. ATP is a nucleotide, a monomeric unit comprising three main components:
-
A nitrogenous base: In ATP, this is adenine, a purine base with a double-ring structure. Adenine's specific arrangement of nitrogen and carbon atoms contributes significantly to its hydrogen bonding capabilities, crucial for interactions within DNA and RNA, and influencing ATP's interaction with enzymes.
-
A pentose sugar: ATP contains ribose, a five-carbon sugar in its furanose form (a five-membered ring). The ribose sugar provides the structural backbone to which the other components attach. The hydroxyl (-OH) groups on the ribose are essential for ATP's reactivity.
-
Phosphate groups: This is where ATP truly differentiates itself. It possesses three phosphate groups linked together by high-energy phosphoanhydride bonds. These phosphate groups are negatively charged, resulting in significant electrostatic repulsion between them. This repulsion stores potential energy, the key to ATP's function as an energy carrier. It's the breaking of these high-energy bonds that releases the energy utilized by cellular processes.
Comparing ATP to Other Molecules
Now let's compare ATP's structure to other molecules to highlight why it's most like a nucleotide:
1. Nucleotides: The Closest Relative
Nucleotides are the building blocks of nucleic acids like DNA and RNA. They share the same basic three-part structure as ATP: a nitrogenous base, a pentose sugar (either ribose or deoxyribose), and one or more phosphate groups. The key difference lies in the number of phosphate groups. While ATP has three, most nucleotides in DNA and RNA have only one. This difference in phosphate number directly impacts energy storage and transfer capabilities. Therefore, ATP's structural similarity to other nucleotides is undeniable.
2. Nucleosides: Missing a Key Component
Nucleosides are simpler molecules composed of only a nitrogenous base and a pentose sugar. They lack the phosphate groups that define ATP's energetic role. While ATP shares the nitrogenous base (adenine) and pentose sugar (ribose) with the nucleoside adenosine, the absence of the phosphate groups makes nucleosides structurally distinct from ATP.
3. Nucleic Acids: Polymers of Nucleotides
Nucleic acids, DNA and RNA, are polymers of nucleotides. While ATP is a nucleotide and therefore a monomeric building block similar to those in nucleic acids, the structural difference lies in its function. Nucleic acids primarily function in storing and transmitting genetic information, while ATP's primary role is energy transfer. The structural similarities are there at the monomeric level, but the polymeric nature of nucleic acids and the monomeric nature of ATP distinguish them.
4. Phospholipids: Similar, but Different
Phospholipids, major components of cell membranes, possess phosphate groups. However, their structure differs significantly from ATP. Phospholipids have a hydrophilic (water-loving) head containing a phosphate group and a glycerol backbone, and two hydrophobic (water-fearing) fatty acid tails. This amphipathic nature is crucial for membrane formation. While both molecules contain phosphate, their overall structures and functions are vastly different.
5. Other Phosphate-Containing Molecules: A Broader Comparison
Many molecules in the cell contain phosphate groups, playing various roles in metabolism and signaling. These include phosphoproteins (proteins with attached phosphate groups), phospholipids (as discussed above), and various metabolic intermediates. However, none of these molecules share the same combination of a nitrogenous base, a pentose sugar, and three phosphate groups in the same arrangement as ATP. The specific combination of components and their arrangement defines ATP's unique structure and function.
The Significance of Structural Similarity to Nucleotides
The close structural resemblance of ATP to nucleotides isn't merely coincidental; it's functionally significant. The nucleotide structure allows ATP to readily interact with enzymes involved in energy metabolism. These enzymes have evolved to recognize and bind to the specific structure of ATP, facilitating the transfer of phosphate groups and the release of energy. The adenine base, ribose sugar, and phosphate groups all play crucial roles in these interactions.
The high-energy phosphoanhydride bonds are critical for energy storage and transfer. The negative charges on the phosphate groups create significant electrostatic repulsion, storing potential energy. Hydrolysis of these bonds, breaking them apart with the addition of water, releases this energy, driving various cellular processes. This hydrolysis often involves transferring a phosphate group to another molecule, changing its conformation and activating it.
ATP's Role as an Energy Carrier: A Deeper Dive
ATP's structural characteristics directly correlate with its function as the primary energy carrier in cells. The high-energy phosphate bonds are central to this role. When ATP is hydrolyzed to ADP (adenosine diphosphate) and inorganic phosphate (Pi), a significant amount of free energy is released. This energy is then coupled to other energetically unfavorable reactions, making them proceed. This coupling is often achieved through the transfer of the phosphate group from ATP to another molecule, a process known as phosphorylation.
Phosphorylation can activate enzymes, change the conformation of proteins, and drive transport across membranes. The energy released from ATP hydrolysis is not directly used to perform cellular work; instead, it is harnessed indirectly through these coupling mechanisms.
Conclusion: ATP's Unique Position
While ATP shares structural similarities with other molecules, its unique combination of a nitrogenous base, a pentose sugar, and three phosphate groups places it firmly within the nucleotide family. The structural similarity to other nucleotides isn't just a superficial resemblance; it's fundamental to ATP's function as the primary energy currency of the cell. The precise arrangement of these components allows for specific interactions with enzymes and facilitates the efficient storage and transfer of energy, crucial for all life processes. The negative charges of the phosphate groups, the ability to undergo hydrolysis, and the specific molecular recognition by enzymes are all consequences of this specific structure. Therefore, the answer remains clear: structurally, ATP is most like a nucleotide.
Latest Posts
Latest Posts
-
What Is The Percentage Of 5 Out Of 6
Mar 28, 2025
-
How Many Inches Is 97 Cm
Mar 28, 2025
-
60 Is What Percent Of 15
Mar 28, 2025
-
3 Is What Percent Of 18
Mar 28, 2025
-
How Many Grams Are In 4 Kilograms
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Structurally Atp Is Most Like Which Type Of Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
