The Bonding Properties Of An Atom Are Determined By Its
Kalali
Mar 14, 2025 · 6 min read
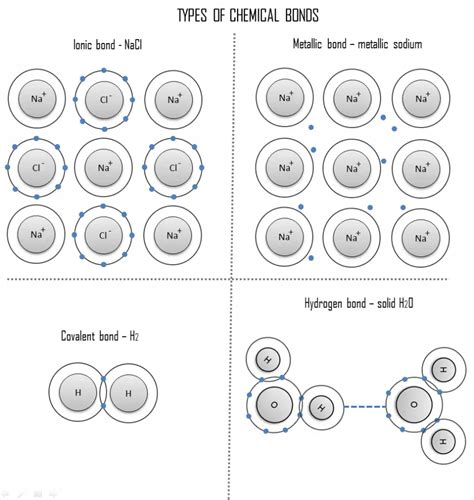
Table of Contents
The Bonding Properties of an Atom are Determined by its Electron Configuration
The fundamental properties of an atom that dictate how it interacts with other atoms, forming molecules and solids, are encapsulated in its electron configuration. This configuration, which describes the arrangement of electrons within the atom's electron shells and subshells, directly governs the atom's bonding properties. Understanding this relationship is crucial in chemistry, materials science, and various other fields. This article will delve into the intricate connection between electron configuration and bonding, exploring the various types of chemical bonds and how they arise from the electron arrangements of the participating atoms.
The Role of Valence Electrons
The key players in chemical bonding are the valence electrons. These are the electrons located in the outermost shell, also known as the valence shell. These electrons are relatively loosely bound to the nucleus and are therefore most readily involved in interactions with other atoms. The number of valence electrons an atom possesses directly determines its bonding capacity and the types of bonds it can form.
Octet Rule and Stability
A fundamental concept in understanding chemical bonding is the octet rule. This rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight electrons in their valence shell, similar to the noble gases. This stable configuration represents a low-energy state, making the atom less reactive. However, it's important to note that the octet rule is a guideline, not a strict law, with exceptions existing, especially for elements beyond the second period.
Types of Chemical Bonds
Based on the electron configuration and the nature of the interactions between valence electrons, atoms can form several types of chemical bonds:
1. Ionic Bonds: Electron Transfer
Ionic bonds arise from the electrostatic attraction between oppositely charged ions. This occurs when one atom, typically a metal with low electronegativity, readily loses valence electrons to become a positively charged cation. The electron(s) are then accepted by another atom, usually a nonmetal with high electronegativity, forming a negatively charged anion. The strong Coulombic forces between the cation and anion result in the formation of an ionic compound.
Example: Sodium (Na) has one valence electron and readily loses it to achieve a stable configuration similar to Neon (Ne). Chlorine (Cl) has seven valence electrons and readily gains one electron to achieve a stable configuration similar to Argon (Ar). The resulting Na⁺ and Cl⁻ ions are held together by a strong ionic bond, forming sodium chloride (NaCl), common table salt.
The electron configuration directly dictates the charge of the ion formed. Atoms with few valence electrons tend to lose electrons to form cations, while atoms with many valence electrons tend to gain electrons to form anions.
2. Covalent Bonds: Electron Sharing
Covalent bonds involve the sharing of valence electrons between two atoms. This sharing occurs when the atoms have similar electronegativities and neither atom readily loses or gains electrons. The shared electrons are attracted to the nuclei of both atoms, creating a stable bond. This type of bond is common among nonmetals.
Example: In the formation of a hydrogen molecule (H₂), each hydrogen atom contributes one electron to the shared pair. This shared pair of electrons is attracted to both hydrogen nuclei, holding the two atoms together in a stable covalent bond.
The number of covalent bonds an atom can form depends on the number of unpaired electrons in its valence shell. For example, carbon (C), with four valence electrons, can form four covalent bonds, as seen in methane (CH₄).
Polar and Nonpolar Covalent Bonds
The nature of the covalent bond can vary depending on the difference in electronegativity between the atoms involved.
-
Nonpolar covalent bonds: These bonds occur when the atoms share electrons equally, typically when the atoms are identical (e.g., H₂) or have very similar electronegativities.
-
Polar covalent bonds: These bonds occur when the atoms share electrons unequally. The atom with higher electronegativity attracts the shared electrons more strongly, creating a partial negative charge (δ⁻) on that atom and a partial positive charge (δ⁺) on the other atom. This creates a dipole moment. Water (H₂O) is a prime example of a molecule with polar covalent bonds.
3. Metallic Bonds: Delocalized Electrons
Metallic bonds are characteristic of metals and are formed due to the delocalized nature of valence electrons. In a metallic solid, the valence electrons are not associated with any particular atom but rather move freely throughout the metallic lattice. This "sea" of delocalized electrons creates strong attractive forces that hold the metal atoms together. This explains the characteristic properties of metals, such as high electrical and thermal conductivity, malleability, and ductility.
The electron configuration of metals, typically with few valence electrons, facilitates the formation of this delocalized electron "sea".
4. Hydrogen Bonds: Special Interactions
Hydrogen bonds are a special type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) and is attracted to another electronegative atom in a nearby molecule. While weaker than ionic or covalent bonds, hydrogen bonds play a crucial role in the properties of many molecules, particularly water, proteins, and DNA.
Factors Influencing Bonding Properties Beyond Electron Configuration
While electron configuration is the primary determinant of bonding properties, several other factors influence the nature and strength of bonds:
-
Electronegativity: The ability of an atom to attract electrons in a chemical bond. The greater the electronegativity difference between two atoms, the more polar the covalent bond or the more likely the formation of an ionic bond.
-
Atomic Size: Larger atoms tend to form weaker bonds due to increased distance between nuclei and valence electrons.
-
Ionization Energy: The energy required to remove an electron from an atom. Lower ionization energies indicate a greater tendency to lose electrons and form cations.
-
Electron Affinity: The energy change that occurs when an electron is added to an atom. Higher electron affinities indicate a greater tendency to gain electrons and form anions.
Predicting Bonding Based on Electron Configuration
By examining the electron configuration of an atom, we can often predict the type of bond it will form and its bonding capacity.
For example:
-
Group 1 elements (alkali metals): These elements have one valence electron and readily lose it to form +1 cations, forming ionic bonds with nonmetals.
-
Group 17 elements (halogens): These elements have seven valence electrons and readily gain one electron to form -1 anions, forming ionic bonds with metals and covalent bonds with other nonmetals.
-
Group 18 elements (noble gases): These elements have a full valence shell and are generally unreactive, showing minimal tendency to form bonds.
-
Transition metals: These elements have variable oxidation states, meaning they can lose different numbers of valence electrons to form various cations, leading to a wider range of possible bond types and complexities in their compounds.
Conclusion
The electron configuration of an atom is the fundamental determinant of its bonding properties. The number and arrangement of valence electrons dictate the type of bonds an atom can form – ionic, covalent, metallic, or a combination thereof. Understanding the relationship between electron configuration and bonding is paramount in comprehending the structure and properties of matter. By analyzing the electron configuration, along with other factors such as electronegativity and atomic size, we can predict the type of chemical bonds that will be formed and gain valuable insights into the behavior and reactivity of elements and compounds. This knowledge underpins advancements in diverse fields, from the design of new materials with specific properties to understanding biological processes at the molecular level.
Latest Posts
Latest Posts
-
How Many Positions Are There In Sex
Jul 02, 2025
-
How Many Minutes Are In 10 Miles
Jul 02, 2025
-
How Many Milliseconds Are In A Day
Jul 02, 2025
-
If Your 16 What Year Were You Born
Jul 02, 2025
-
Ten Thousand 2 Hundrad And 14 How To Writew Numercally
Jul 02, 2025
Related Post
Thank you for visiting our website which covers about The Bonding Properties Of An Atom Are Determined By Its . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.