The Carbon Atoms In Saturated Hydrocarbons
Kalali
Mar 19, 2025 · 6 min read
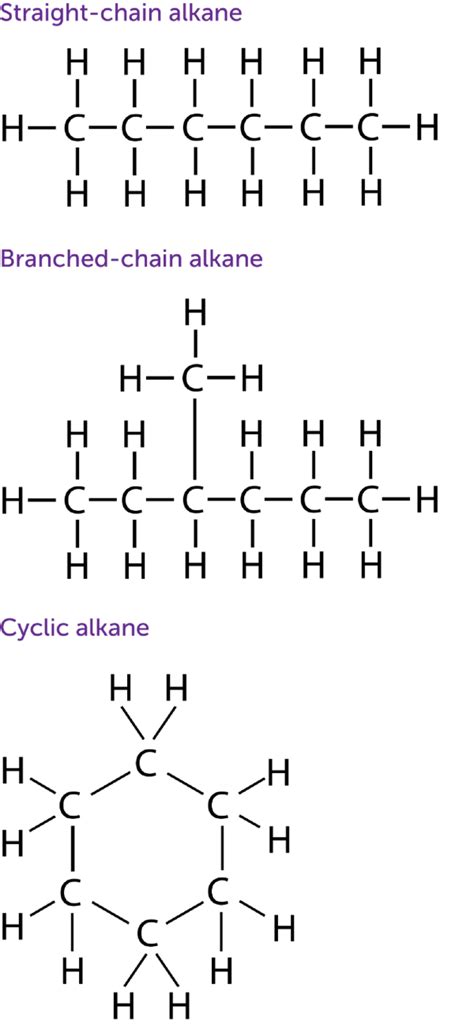
Table of Contents
Delving Deep into the Carbon Atoms of Saturated Hydrocarbons
Saturated hydrocarbons, also known as alkanes, form the bedrock of organic chemistry. Their seemingly simple structure – carbon atoms bonded solely through single bonds to hydrogen atoms – belies a rich tapestry of chemical properties and applications. Understanding the behavior and characteristics of the carbon atoms within these molecules is crucial to grasping their role in everything from fuels to plastics. This comprehensive exploration will delve deep into the fascinating world of carbon atoms in saturated hydrocarbons, covering their bonding, structure, properties, and applications.
The Fundamental Building Block: Carbon's Tetrahedral Geometry
At the heart of every saturated hydrocarbon lies the carbon atom. Carbon's unique ability to form four strong covalent bonds is the fundamental reason for the existence of such a vast array of organic compounds. In saturated hydrocarbons, each carbon atom is sp³ hybridized. This means that one s orbital and three p orbitals combine to form four equivalent sp³ hybrid orbitals, each oriented towards the corners of a tetrahedron. This tetrahedral geometry is crucial in defining the three-dimensional structure and properties of alkanes.
Understanding sp³ Hybridization
The sp³ hybridization is not merely a theoretical concept; it has tangible consequences. The tetrahedral arrangement of the four sp³ hybrid orbitals maximizes the distance between the electron pairs, minimizing electron-electron repulsion and leading to the most stable configuration. This directly influences the bond angles within the molecule. In a perfect tetrahedron, the bond angle is 109.5°. While minor deviations from this ideal angle can occur due to steric hindrance (repulsion between bulky groups), the tetrahedral geometry remains a dominant feature.
The Role of Sigma Bonds
Each of the four sp³ hybrid orbitals forms a strong sigma (σ) bond. Sigma bonds are characterized by head-on overlap of atomic orbitals, resulting in high electron density concentrated along the internuclear axis. The strength and stability of these sigma bonds are key reasons for the relative inertness of saturated hydrocarbons under normal conditions. They require significant energy to break, leading to their lower reactivity compared to unsaturated hydrocarbons (alkenes and alkynes).
Structural Variations: From Methane to Complex Alkanes
The simplest saturated hydrocarbon is methane (CH₄), with a single carbon atom at the center bonded to four hydrogen atoms. As we move to larger alkanes, the complexity increases. The carbon atoms can form chains – straight chains (normal alkanes) or branched chains (branched alkanes) – leading to a vast number of structural isomers.
Straight-Chain Alkanes: A Simple Start
Straight-chain alkanes, such as ethane (C₂H₆), propane (C₃H₈), and butane (C₄H₁₀), provide a straightforward illustration of the carbon-carbon single bonds. Each carbon atom maintains its tetrahedral geometry, with the carbon-carbon bonds also exhibiting a bond angle close to 109.5°. The properties of these alkanes change gradually as the chain length increases, showcasing the effect of increasing molecular size.
Branched-Chain Alkanes: Introducing Isomers
The introduction of branching significantly increases the number of possible isomers. Isomers are molecules with the same molecular formula but different structural arrangements. For example, butane (C₄H₁₀) exists as two isomers: n-butane (a straight chain) and isobutane (a branched chain). This branching affects the molecule's physical properties, such as boiling point and melting point. Branched-chain alkanes generally have lower boiling points than their straight-chain counterparts due to a reduced surface area for intermolecular interactions.
Properties Influenced by Carbon Atom Arrangement
The arrangement of carbon atoms directly affects a variety of physical and chemical properties of saturated hydrocarbons:
Boiling Point and Melting Point
As the number of carbon atoms increases, the boiling and melting points of alkanes generally increase. This is due to stronger London dispersion forces between larger molecules. Branching, however, lowers the boiling point because it reduces the surface area available for intermolecular interactions.
Density
Alkanes are generally less dense than water. Their density increases with increasing molecular weight, but they remain less dense than water throughout the homologous series.
Solubility
Alkanes are nonpolar molecules, and thus are insoluble in polar solvents like water. They are, however, soluble in nonpolar solvents such as other hydrocarbons.
Reactivity
Saturated hydrocarbons are relatively unreactive due to the strong sigma bonds. They do not readily undergo addition reactions like alkenes and alkynes. Their primary reactions are combustion (burning in oxygen) and substitution reactions (replacement of a hydrogen atom with another atom or group).
Applications of Saturated Hydrocarbons: A Diverse Range
The widespread use of saturated hydrocarbons stems directly from their properties. Their abundance, relative inertness, and ability to undergo combustion make them invaluable in various industries:
Fuels: Powering Our World
Alkanes are the primary components of natural gas (primarily methane) and petroleum (a mixture of various alkanes). They serve as crucial fuels for transportation, heating, and electricity generation. The combustion of alkanes releases a significant amount of energy, making them efficient sources of power.
Plastics and Polymers: Shaping Modern Life
Many plastics are derived from alkanes through processes like cracking and polymerization. Polyethylene, polypropylene, and other polymers are extensively used in packaging, construction, and manufacturing diverse products. The flexibility and durability of these materials are directly linked to the properties of the constituent carbon-carbon single bonds.
Solvents and Lubricants: Essential Industrial Chemicals
Certain alkanes are used as solvents in various industrial processes. Their nonpolar nature makes them suitable for dissolving nonpolar substances. Higher molecular weight alkanes serve as lubricants, reducing friction between moving parts in machinery.
Further Exploration: Conformation and Isomerism
The discussion above only scratches the surface of the complexities associated with carbon atoms in saturated hydrocarbons. Further exploration would necessitate a deeper dive into conformational isomerism and stereoisomerism.
Conformational Isomers: Rotations Around Bonds
The rotation around carbon-carbon single bonds allows for various conformations of a molecule. These conformations differ in their energy levels, with some being more stable than others. The study of conformations is crucial for understanding the shapes and reactions of alkanes.
Stereoisomers: Beyond Structural Differences
Stereoisomers are molecules with the same molecular formula and connectivity but different spatial arrangements of atoms. While not directly related to the sp³ hybridization, understanding stereoisomerism is crucial for comprehending the behavior of more complex alkanes and their derivatives.
Conclusion: A Foundation of Organic Chemistry
The carbon atoms in saturated hydrocarbons are more than just simple building blocks; they are the fundamental units that underpin a vast array of organic molecules and their associated properties. Understanding their sp³ hybridization, tetrahedral geometry, and the impact on molecular shape and reactivity is crucial for anyone studying organic chemistry or exploring the numerous applications of alkanes in our daily lives. From fuels powering our world to the plastics shaping our environment, the significance of these seemingly simple molecules cannot be overstated. Future research will likely continue to uncover new applications and deeper insights into the fascinating world of saturated hydrocarbons and their constituent carbon atoms.
Latest Posts
Latest Posts
-
What Is 11 25 As A Percentage
Mar 19, 2025
-
Cuanto Es El 2 Por Ciento De 1000
Mar 19, 2025
-
40 Of 20 Is What Number
Mar 19, 2025
-
Cuanto Es El 30 De 300
Mar 19, 2025
-
Cuanto Es 200 Miligramos De Agua
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about The Carbon Atoms In Saturated Hydrocarbons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
