The Only Metalloid With 3 Valence Electrons
Kalali
Mar 14, 2025 · 6 min read
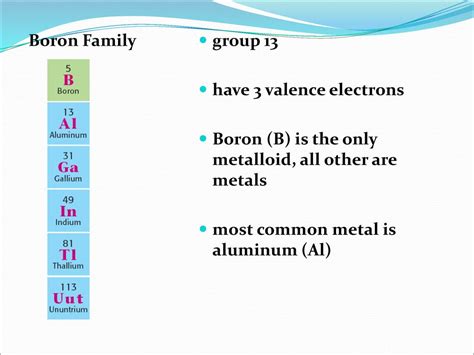
Table of Contents
The Only Metalloid with 3 Valence Electrons: Boron – A Deep Dive
Boron, a fascinating element residing on the precipice between metals and nonmetals, holds a unique distinction: it's the only metalloid with three valence electrons. This seemingly simple fact underpins boron's remarkable properties, its diverse applications, and the ongoing research surrounding its potential. Understanding boron's electronic structure is key to unlocking its multifaceted nature. This comprehensive article will explore boron's unique characteristics, its chemical behavior, its diverse applications, and the ongoing research into its potential.
Understanding Valence Electrons and Metalloids
Before delving into the specifics of boron, let's establish a clear understanding of the terms "valence electrons" and "metalloids."
Valence Electrons: The Key to Reactivity
Valence electrons are the electrons located in the outermost shell of an atom. These electrons are crucial because they determine an atom's chemical behavior and its ability to form bonds with other atoms. Atoms strive to achieve a stable electron configuration, often by gaining, losing, or sharing valence electrons. The number of valence electrons dictates the bonding capacity of an element and its position in the periodic table.
Metalloids: Bridging the Gap
Metalloids, also known as semimetals, occupy a fascinating middle ground in the periodic table. They possess properties intermediate between those of metals and nonmetals. This means they exhibit characteristics of both groups, leading to a diverse range of applications. Unlike metals, they are typically poor conductors of electricity, although their conductivity can be significantly improved under specific conditions. Unlike nonmetals, they may exhibit some metallic luster. Their behavior is highly dependent on various factors, including temperature and pressure.
Boron: The Unique Metalloid
Boron, with its atomic number 5, is uniquely positioned in the periodic table. Its electronic configuration ([He] 2s²2p¹) reveals that it possesses three valence electrons. This is what distinguishes it from all other metalloids. Silicon, germanium, arsenic, antimony, and tellurium, all have more than three valence electrons. This unique electronic structure is responsible for boron's distinctive chemical and physical properties.
Boron's Physical Properties: A Unique Blend
Boron's physical properties reflect its metalloid nature. It exists in several allotropic forms, each with slightly different properties. Amorphous boron is a dark brown powder, while crystalline boron is extremely hard, brittle, and exhibits a metallic luster despite its semiconducting behavior. Its melting point is exceptionally high, reflecting strong covalent bonds within its crystal structure. It's a poor conductor of electricity at room temperature but becomes a better conductor at higher temperatures, displaying semiconducting behavior.
Boron's Chemical Behavior: The Influence of Three Valence Electrons
Boron's three valence electrons significantly influence its chemical behavior. It readily forms covalent bonds, sharing its three electrons with other atoms. This is in contrast to metals, which tend to lose electrons to form positive ions. Boron's chemistry is complex and diverse, leading to the formation of a wide range of compounds. It readily combines with elements such as oxygen, hydrogen, nitrogen, and halogens.
Boron Compounds: A Diverse Family
Boron forms an extensive range of compounds, showcasing its versatile chemical behavior. Some notable examples include:
- Boron oxides: Boric oxide (B₂O₃) is a crucial boron compound, used extensively in glass manufacturing.
- Boron halides: Compounds like boron trifluoride (BF₃) are powerful Lewis acids, frequently used as catalysts in organic chemistry.
- Boranes: These compounds contain only boron and hydrogen and exhibit unique bonding arrangements. They are known for their interesting structures and reactivity.
- Boron nitride: Similar in structure to graphite, boron nitride (BN) exists in various forms, including hexagonal boron nitride (h-BN) which is known for its exceptional thermal and chemical resistance. Cubic boron nitride (c-BN) is incredibly hard, second only to diamond.
- Borates: These are salts containing boron and oxygen, and often other elements. They find applications in detergents, fertilizers, and other industrial processes.
Applications of Boron: From Glass to Nuclear Reactors
Boron's unique properties have led to its widespread use across various industries. Its applications range from everyday materials to high-tech components. This versatility stems from its ability to form strong covalent bonds, its semiconducting properties, and its ability to act as a neutron absorber.
Boron in Everyday Materials
- Glass: Boron is a crucial ingredient in borosilicate glass, known for its resistance to thermal shock. This type of glass is commonly used in laboratory glassware and ovenware.
- Ceramics: Boron contributes to the strength and durability of certain ceramics.
- Detergents: Borates are used as water softeners and builders in laundry detergents.
- Fertilizers: Boron is an essential micronutrient for plant growth, and boron-containing fertilizers are widely used in agriculture.
Boron in High-Tech Applications
- Semiconductors: Boron's semiconducting properties make it valuable in the electronics industry, specifically in doping silicon to create p-type semiconductors.
- Nuclear Reactors: Boron's ability to absorb neutrons makes it crucial in nuclear reactors as a control rod material, regulating the chain reaction.
- High-strength materials: Boron fibers are used in advanced composite materials for their high strength-to-weight ratio, finding applications in aerospace and defense industries.
- Medicine: Boron compounds are used in Boron Neutron Capture Therapy (BNCT), a type of cancer treatment.
- Flame retardants: Boron compounds are used as flame retardants in various materials due to their ability to disrupt combustion processes.
Ongoing Research and Future Potential
Despite its established applications, research into boron continues to unveil new possibilities and enhance existing applications.
Exploring Boron's Unique Bonding
Scientists are continually exploring the intricacies of boron's bonding, aiming to design new materials with tailored properties. The unique bonding characteristics of boron can lead to the creation of novel materials with exceptional strength, conductivity, or catalytic activity.
Boron Nanomaterials: A Frontier of Innovation
The development of boron-based nanomaterials, such as boron nitride nanotubes and boron clusters, is a rapidly evolving field. These materials exhibit remarkable properties, opening up exciting possibilities in various applications, including electronics, medicine, and energy storage.
Boron in Energy Technologies
Boron's potential in energy technologies is being actively investigated. Research is focused on developing boron-based materials for batteries, fuel cells, and solar energy applications. Its ability to facilitate efficient charge transfer and its high energy density make it an attractive candidate for energy storage solutions.
Boron's Role in Catalysis
Boron's unique chemical behavior makes it a promising catalyst in various chemical processes. Researchers are exploring the use of boron-based catalysts to improve the efficiency and selectivity of chemical reactions, leading to greener and more sustainable industrial processes.
Conclusion: The Enduring Importance of Boron
Boron, the only metalloid with three valence electrons, is a truly remarkable element. Its unique electronic structure underpins its diverse properties and its widespread applications. From everyday materials to high-tech components, boron plays a vital role in modern society. Ongoing research continues to unveil new facets of boron's potential, promising exciting advancements in various fields, from energy technologies to medicine. Understanding boron's unique characteristics is crucial for harnessing its full potential and shaping a future driven by innovation. The continued investigation into its properties and applications ensures that boron will remain an element of significant scientific and technological importance for years to come. Its unique position in the periodic table, with its distinct three valence electrons, firmly secures its place as a key player in the realm of materials science and beyond.
Latest Posts
Latest Posts
-
How Many Feet Is 40 M
Mar 14, 2025
-
How Many Miles Is A 6k
Mar 14, 2025
-
How Many Ml In 32 Ounces
Mar 14, 2025
-
How Many Ounces In 12 Cups
Mar 14, 2025
-
What Is 39 Celsius In Fahrenheit
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about The Only Metalloid With 3 Valence Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
