The Ratio Of Atoms In Hcl Is
Kalali
Mar 22, 2025 · 5 min read
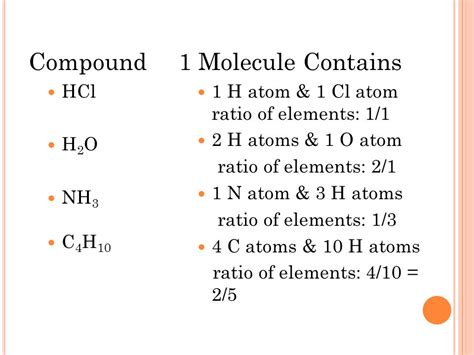
Table of Contents
The Ratio of Atoms in HCl: A Deep Dive into Chemical Stoichiometry
The seemingly simple molecule HCl, hydrogen chloride, provides a fantastic entry point into understanding chemical stoichiometry – the quantitative relationship between reactants and products in a chemical reaction. While the ratio of atoms in HCl might appear obvious at first glance, exploring this seemingly simple concept opens doors to a deeper understanding of chemical formulas, molar masses, and the fundamental building blocks of matter. This article will delve into the atomic ratio in HCl, its implications, and how this understanding extends to more complex chemical scenarios.
Understanding the Chemical Formula of HCl
The chemical formula HCl concisely represents the composition of hydrogen chloride. Each capital letter represents a different chemical element, and the number following the element (or an implied "1" if no number is present) indicates the number of atoms of that element present in one molecule of the compound. Therefore, HCl tells us that one molecule of hydrogen chloride contains one atom of hydrogen (H) and one atom of chlorine (Cl). This 1:1 ratio is crucial in understanding its properties and reactions.
The Significance of the 1:1 Ratio
This simple 1:1 atomic ratio forms the basis for many calculations and predictions in chemistry. It allows us to determine:
-
Molar Mass: Knowing the atomic masses of hydrogen (approximately 1 g/mol) and chlorine (approximately 35.5 g/mol), we can calculate the molar mass of HCl as 1 + 35.5 = 36.5 g/mol. This means that one mole of HCl (approximately 6.022 x 10²³ molecules) weighs 36.5 grams.
-
Percent Composition: The 1:1 ratio allows us to easily calculate the percentage by mass of each element in HCl. Hydrogen contributes approximately 1/36.5 * 100% ≈ 2.74%, while chlorine contributes approximately 35.5/36.5 * 100% ≈ 97.26%.
-
Reaction Stoichiometry: In chemical reactions involving HCl, the 1:1 ratio dictates the proportions in which it reacts with other substances. For example, in the reaction between HCl and sodium hydroxide (NaOH) to produce sodium chloride (NaCl) and water (H₂O):
HCl + NaOH → NaCl + H₂O
The balanced equation shows that one mole of HCl reacts with one mole of NaOH. This direct correlation stems directly from the atomic ratio within the HCl molecule.
Extending the Concept: Beyond Simple Ratios
While HCl exemplifies a simple 1:1 atomic ratio, many other compounds exhibit more complex ratios. Understanding the atomic ratios in these compounds relies on the same fundamental principles.
Examples of Different Atomic Ratios
-
Water (H₂O): Water has a ratio of 2:1 for hydrogen to oxygen. Two hydrogen atoms bond with one oxygen atom to form a single water molecule. This ratio directly impacts its properties, such as its polarity and its role as a universal solvent.
-
Carbon Dioxide (CO₂): Carbon dioxide has a ratio of 1:2 for carbon to oxygen. One carbon atom bonds with two oxygen atoms. This ratio is crucial in understanding its role in the greenhouse effect and its behavior in various chemical reactions.
-
Methane (CH₄): Methane exhibits a 1:4 ratio of carbon to hydrogen. One carbon atom bonds with four hydrogen atoms. This ratio dictates its energy content and its contribution to climate change.
-
Glucose (C₆H₁₂O₆): Glucose, a simple sugar, showcases a more complex ratio of 6:12:6 for carbon, hydrogen, and oxygen, respectively. This ratio determines its energy content and its role in biological processes.
Understanding these ratios is essential for various applications, from calculating the amount of reactants needed in a chemical reaction to determining the empirical formula of a compound from experimental data.
Determining Atomic Ratios from Empirical and Molecular Formulas
The atomic ratios within a molecule are directly reflected in its chemical formula. Two types of formulas are commonly used:
-
Empirical Formula: The empirical formula represents the simplest whole-number ratio of atoms in a compound. For HCl, the empirical formula is HCl, reflecting the 1:1 ratio. For glucose (C₆H₁₂O₆), the empirical formula is CH₂O, showing the simplified 1:2:1 ratio.
-
Molecular Formula: The molecular formula represents the actual number of atoms of each element in one molecule of the compound. For HCl, the molecular formula is also HCl. However, for compounds like glucose, the molecular formula (C₆H₁₂O₆) differs from the empirical formula (CH₂O), indicating the presence of multiple units of the empirical formula within the molecule.
Determining the empirical and molecular formulas of a compound often involves experimental techniques like combustion analysis and mass spectrometry. These techniques provide data on the mass percentages of each element in the compound, allowing chemists to determine the atomic ratios and subsequently the formulas.
Applications of Understanding Atomic Ratios
The understanding of atomic ratios extends far beyond simple calculations; it underpins numerous applications in various scientific disciplines:
Chemistry
-
Chemical Reactions: Predicting the quantities of reactants and products in chemical reactions relies heavily on the atomic ratios within the molecules involved. Balancing chemical equations accurately requires a complete understanding of these ratios.
-
Titrations: In titrations, the atomic ratio between the titrant and the analyte helps determine the concentration of an unknown solution.
-
Stoichiometric Calculations: Numerous stoichiometric calculations, such as determining the limiting reactant, theoretical yield, and percent yield, rely on the precise atomic ratios in chemical formulas.
Biology
-
Biochemistry: Understanding the atomic ratios in biological molecules like proteins, carbohydrates, and lipids is crucial for comprehending their structure, function, and metabolism.
-
Genetics: The ratios of different bases in DNA and RNA are fundamental to understanding genetic codes and replication processes.
Environmental Science
- Pollution Control: Understanding the atomic ratios in pollutants helps determine their sources and develop strategies for effective pollution control.
Materials Science
- Material Synthesis: Controlling the atomic ratios of elements in materials synthesis allows for the creation of materials with desired properties.
Conclusion: The Foundation of Chemistry
The seemingly simple ratio of atoms in HCl – 1:1 – serves as a fundamental building block in understanding chemical stoichiometry. This concept extends far beyond a single molecule, underpinning complex chemical calculations, reaction predictions, and a deeper appreciation for the composition and behavior of matter. By mastering the concept of atomic ratios, we unlock a deeper understanding of the world around us, from the smallest molecules to the most complex biological systems and materials. The ability to accurately determine and apply these ratios is an essential skill for any chemist, biologist, or material scientist, enabling breakthroughs in numerous fields of science and technology.
Latest Posts
Latest Posts
-
How Many Pounds In 58 Kg
Mar 22, 2025
-
Como Se Escribe El 0 En Numero Romano
Mar 22, 2025
-
Compare And Contrast The Inner Planets And The Outer Planets
Mar 22, 2025
-
How Many Feet Is 196 Inches
Mar 22, 2025
-
How To Convert A Square Root To A Decimal
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about The Ratio Of Atoms In Hcl Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
