The Types Of Bonds Found In Nucleic Acids Are
Kalali
Mar 26, 2025 · 6 min read
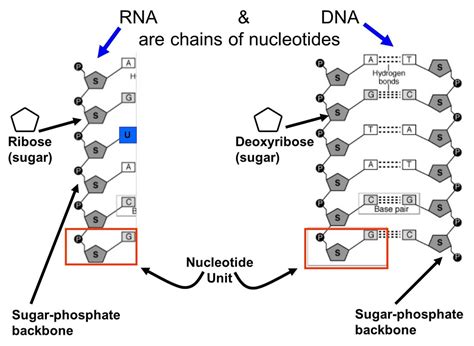
Table of Contents
The Types of Bonds Found in Nucleic Acids Are... A Deep Dive
Nucleic acids, the fundamental building blocks of life, are complex polymers responsible for storing and transmitting genetic information. Understanding the types of bonds that hold these molecules together is crucial to comprehending their structure, function, and overall biological significance. This comprehensive exploration delves into the various bonds present in nucleic acids, examining their properties, roles, and the implications of their presence for the stability and dynamic behavior of DNA and RNA.
The Backbone: Phosphodiester Bonds – The Glue Holding Nucleic Acids Together
The primary structure of nucleic acids, whether DNA or RNA, is characterized by a sugar-phosphate backbone. This backbone is formed by the phosphodiester bonds that link the 3'-carbon of one sugar molecule to the 5'-carbon of the adjacent sugar molecule. This linkage is a key defining feature of nucleic acids, creating a directional polarity (5' to 3') crucial for many biological processes.
The Chemistry of Phosphodiester Bonds
A phosphodiester bond is a covalent bond formed between a phosphate group and two hydroxyl groups on two separate sugar molecules. Specifically, the phosphate group's two hydroxyl groups react with the 3'-hydroxyl of one sugar and the 5'-hydroxyl of another. This reaction involves the elimination of a water molecule, a dehydration synthesis, creating a strong and stable linkage. The negatively charged phosphate groups in the backbone contribute significantly to the overall negative charge of nucleic acids. This negative charge plays important roles in interactions with proteins and ions and influences the secondary and tertiary structures of DNA and RNA.
Significance of Phosphodiester Bonds for Nucleic Acid Structure and Function
The phosphodiester bonds' strength is critical to maintaining the structural integrity of nucleic acids. This stability allows the genetic information encoded within the sequence of nucleotides to be preserved and accurately replicated. The consistent nature of the phosphodiester bond ensures the uniformity of the backbone, contributing to the double helix structure of DNA and the various folded structures adopted by RNA. The directionality of the backbone, established by the 5' to 3' linkage, is vital for processes such as DNA replication and transcription, where the synthesis of new nucleic acid strands occurs in a specific direction.
The Rungs of the Ladder: Hydrogen Bonds – Dictating Base Pairing Specificity
While the phosphodiester bonds form the backbone, the hydrogen bonds connect the nitrogenous bases, forming the "rungs" of the DNA double helix or the folded structures of RNA. These bonds, although individually weaker than covalent bonds, are collectively responsible for the remarkable specificity of base pairing and the overall stability of nucleic acid structures.
Base Pairing Rules: A-T and G-C
In DNA, adenine (A) forms two hydrogen bonds with thymine (T), while guanine (G) forms three hydrogen bonds with cytosine (C). This specific base pairing, known as Chargaff's rules, is fundamental to the double helix structure and the accurate replication of DNA. The strength of hydrogen bonding between base pairs influences the stability of the DNA double helix, with G-C base pairs being slightly stronger due to the presence of three hydrogen bonds compared to the two in A-T base pairs. This difference in bond strength is reflected in the melting temperature of DNA, where regions richer in G-C base pairs require higher temperatures to separate the strands.
RNA Base Pairing: A-U and G-C
RNA, while structurally similar to DNA in its backbone, uses uracil (U) instead of thymine (T). Therefore, in RNA, adenine (A) forms two hydrogen bonds with uracil (U), while guanine (G) still forms three hydrogen bonds with cytosine (C). The variation in base pairing is a reflection of RNA's diverse functional roles, enabling its adoption of complex three-dimensional structures crucial for its catalytic activity and regulatory functions.
Importance of Hydrogen Bonds for Nucleic Acid Function
Hydrogen bonds, despite their relative weakness, are crucial for the following:
- Base Pairing Specificity: The precise nature of hydrogen bonding ensures accurate replication of DNA and precise transcription of genetic information into RNA.
- DNA Double Helix Stability: The cumulative effect of many hydrogen bonds contributes significantly to the stability of the double helix, protecting the genetic information from damage.
- RNA Secondary and Tertiary Structure: Hydrogen bonds play a crucial role in the folding of RNA molecules into complex three-dimensional structures. These structures are essential for RNA's diverse functions, including catalysis (ribozymes) and gene regulation.
- DNA-Protein Interactions: Hydrogen bonds are also involved in the interactions between DNA and proteins, such as transcription factors that regulate gene expression.
Beyond the Backbone and Base Pairs: Other Important Bonds in Nucleic Acids
While phosphodiester and hydrogen bonds are the most prominent, several other types of bonds play important roles in shaping the three-dimensional structure and functionality of nucleic acids.
Van der Waals Forces: Weak Interactions with Significant Cumulative Effects
Van der Waals forces are weak, short-range attractive forces that arise from temporary fluctuations in electron distribution within molecules. Although individually weak, these forces contribute significantly to the overall stability of nucleic acid structures. They are particularly important in stabilizing base stacking within the DNA double helix and influencing the packing of DNA and RNA molecules.
Hydrophobic Interactions: Base Stacking and Structural Organization
Hydrophobic interactions, driven by the tendency of nonpolar molecules to aggregate in an aqueous environment, play a critical role in base stacking within the DNA double helix and RNA secondary structures. The hydrophobic nitrogenous bases tend to cluster together in the interior of the helix, away from the surrounding water molecules, further stabilizing the structure.
Ionic Interactions: Influence of the Phosphate Backbone
The negatively charged phosphate backbone of nucleic acids readily participates in ionic interactions with positively charged ions, such as magnesium (Mg²⁺) and other cations. These interactions are crucial for stabilizing nucleic acid structures, particularly in neutralizing the negative charges of the phosphate backbone and influencing the overall conformation of the molecule. They also play a vital role in interactions with proteins and other cellular components.
Implications of Bond Types for Nucleic Acid Function
The interplay between the various types of bonds in nucleic acids determines their structural properties and biological function. The strength and specificity of phosphodiester bonds guarantee the integrity of the genetic code, while the dynamic nature of hydrogen bonds allows for processes like DNA replication and transcription. The weaker forces, such as van der Waals interactions and hydrophobic interactions, contribute subtly but significantly to the overall stability and higher-order structure of these molecules. The influence of ionic interactions further underscores the importance of the cellular environment in shaping the structure and function of nucleic acids.
Conclusion: A Symphony of Bonds
The intricate dance of phosphodiester bonds, hydrogen bonds, van der Waals forces, hydrophobic interactions, and ionic interactions creates the remarkable structures and functions of nucleic acids. Understanding these diverse bonds, their strengths, and their dynamic interplay is essential to appreciating the complexity and elegance of the molecular machinery of life. This complex interplay is not merely a structural feature; it is a critical component that allows for the accurate storage, replication, and expression of genetic information, processes that are fundamental to the existence and propagation of life itself. Future research into the detailed nature of these bonds and their interactions will undoubtedly reveal further intricacies in the fascinating world of nucleic acids and their crucial roles in biological systems.
Latest Posts
Latest Posts
-
What Is 0 Degrees In Fahrenheit To Celsius
Mar 29, 2025
-
The Is The Fundamental Unit Of Life
Mar 29, 2025
-
How Many Cm Is 4 6
Mar 29, 2025
-
100 Of 500 Is What Percent
Mar 29, 2025
-
How Many Grams Is A 8th Of An Ounce
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about The Types Of Bonds Found In Nucleic Acids Are . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
