Under What Conditions Are Gases Most Likely To Behave Ideally
Kalali
Mar 16, 2025 · 5 min read
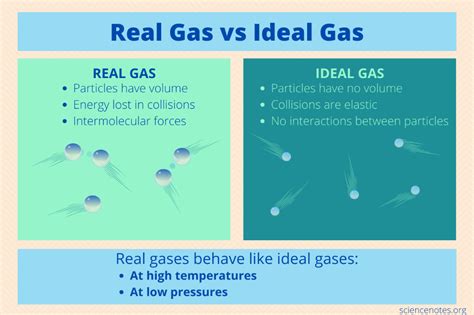
Table of Contents
- Under What Conditions Are Gases Most Likely To Behave Ideally
- Table of Contents
- Under What Conditions Are Gases Most Likely to Behave Ideally?
- The Ideal Gas Law: A Foundation for Understanding
- Conditions Favoring Ideal Gas Behavior
- 1. High Temperature: Minimizing Intermolecular Forces
- 2. Low Pressure: Minimizing Molecular Volume
- 3. Non-Polar Gases: Reducing Attractive Forces
- 4. Monatomic Gases: Simpler Interactions
- Deviations from Ideal Gas Behavior: Real Gases in Action
- Practical Applications and Implications
- Conclusion: A Balancing Act of Temperature, Pressure, and Molecular Properties
- Latest Posts
- Latest Posts
- Related Post
Under What Conditions Are Gases Most Likely to Behave Ideally?
Understanding the behavior of gases is crucial in numerous scientific fields, from chemistry and physics to engineering and atmospheric science. While real gases exhibit complex interactions, the concept of an ideal gas provides a simplified model that accurately predicts behavior under specific conditions. This article delves into the factors influencing ideal gas behavior and explores the conditions under which gases are most likely to adhere to the ideal gas law.
The Ideal Gas Law: A Foundation for Understanding
The ideal gas law, PV = nRT, is a fundamental equation in physical chemistry. It relates the pressure (P), volume (V), number of moles (n), and temperature (T) of an ideal gas through the ideal gas constant (R). This law assumes that gas particles are infinitesimally small points with no volume and that there are no attractive or repulsive forces between them. These assumptions, of course, are not entirely true for real gases, but the ideal gas law serves as a valuable approximation under certain circumstances.
Conditions Favoring Ideal Gas Behavior
Several factors significantly influence how closely a gas behaves like an ideal gas. These conditions can be broadly categorized as:
1. High Temperature: Minimizing Intermolecular Forces
At high temperatures, gas particles possess substantial kinetic energy. This high kinetic energy overcomes the weak intermolecular forces (like van der Waals forces) that exist between real gas molecules. When these forces are negligible, the assumption of no intermolecular interactions in the ideal gas model becomes more accurate. The particles move rapidly and independently, minimizing the effect of attractions or repulsions on the overall behavior of the gas.
Think of it like this: Imagine a group of people at a lively party (high temperature). They're moving around so much that they barely interact with each other beyond brief, fleeting collisions. This is analogous to gas particles at high temperatures, largely unaffected by intermolecular forces.
2. Low Pressure: Minimizing Molecular Volume
Low pressure means the gas molecules are far apart. This condition minimizes the volume occupied by the gas molecules themselves relative to the total volume of the container. The ideal gas law assumes that the gas molecules have negligible volume; at low pressures, this assumption is reasonably valid. With ample space between particles, the contribution of the individual molecular volumes becomes insignificant compared to the overall container volume.
Analogy time again: Imagine the same group of people at a very large party (low pressure). They have so much space to move around that their individual body sizes become insignificant compared to the vast area they occupy. This mirrors how at low pressures, the volume of gas molecules becomes negligible compared to the container's volume.
3. Non-Polar Gases: Reducing Attractive Forces
The strength of intermolecular forces depends heavily on the polarity of the gas molecules. Non-polar gases, such as nitrogen (N₂), oxygen (O₂), and methane (CH₄), have weak London dispersion forces. These weak forces are easily overcome at moderate temperatures and pressures, making these gases more likely to behave ideally.
Polar gases, on the other hand, possess stronger dipole-dipole interactions or hydrogen bonds. These stronger forces cause greater deviations from ideal gas behavior, even at relatively high temperatures and low pressures. The attractions between molecules significantly influence the overall pressure and volume.
4. Monatomic Gases: Simpler Interactions
Monatomic gases, such as helium (He), neon (Ne), and argon (Ar), consist of single atoms. They have the simplest intermolecular interactions, primarily London dispersion forces, which are generally weaker than those found in polyatomic gases. This simplicity contributes to their closer adherence to the ideal gas law under a broader range of conditions compared to polyatomic gases.
Deviations from Ideal Gas Behavior: Real Gases in Action
While the conditions mentioned above promote ideal gas behavior, real gases inevitably deviate from the ideal gas law. These deviations are primarily due to:
-
Intermolecular forces: Attractive forces between molecules cause the actual pressure to be less than that predicted by the ideal gas law. Repulsive forces at high pressures cause the actual pressure to be greater than predicted.
-
Molecular volume: The finite volume of gas molecules is ignored in the ideal gas law. At high pressures, the volume occupied by the molecules themselves becomes significant, leading to deviations from the ideal behavior.
The van der Waals equation is a modified version of the ideal gas law that accounts for these deviations by introducing two correction factors: one for intermolecular attractions (a) and one for molecular volume (b).
Practical Applications and Implications
Understanding the conditions under which gases behave ideally is crucial in many applications:
-
Chemical Engineering: Ideal gas calculations are used in designing and optimizing industrial processes involving gases, such as reaction vessels, distillation columns, and pipelines. While real gas behavior must be considered for accurate results under extreme conditions, the ideal gas law offers a simplified initial approximation.
-
Atmospheric Science: The ideal gas law is applied to model the behavior of gases in the Earth's atmosphere, particularly at relatively high altitudes and low pressures. This is essential for predicting weather patterns and understanding atmospheric phenomena.
-
Aerospace Engineering: Ideal gas models are used in designing and analyzing aircraft and rocket engines, where gas behavior at various pressures and temperatures is crucial.
-
Medical Applications: Understanding gas behavior is important in respiratory therapy and other medical applications involving gas exchange in the body.
Conclusion: A Balancing Act of Temperature, Pressure, and Molecular Properties
The ideal gas law is a powerful tool for understanding gas behavior, but its application relies on recognizing its limitations. Gases are most likely to behave ideally under conditions of high temperature, low pressure, when they are non-polar, and when they are monatomic. Deviation from these ideal conditions increases the influence of intermolecular forces and molecular volume, leading to real gas behavior. While the ideal gas law may not perfectly describe all gas behaviors, it serves as a valuable foundation for understanding the complex world of gas dynamics and its multifaceted applications. Understanding the conditions under which gases exhibit ideal behavior is crucial for accurate predictions and effective applications across diverse scientific and engineering disciplines. By considering temperature, pressure, molecular properties, and the limitations of the model, we can apply the ideal gas law effectively and appreciate its utility in diverse applications.
Latest Posts
Latest Posts
-
How Many Mm In A Litre
Mar 18, 2025
-
140 Centimeters Is How Many Inches
Mar 18, 2025
-
What Percent Is 5 Out Of 6
Mar 18, 2025
-
How Many Feet Are In 200 Meters
Mar 18, 2025
-
Solving Simple Equations With Two Variables Word Pro
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Under What Conditions Are Gases Most Likely To Behave Ideally . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
