Water Is Always A Product In What Type Of Reaction
Kalali
Mar 31, 2025 · 5 min read
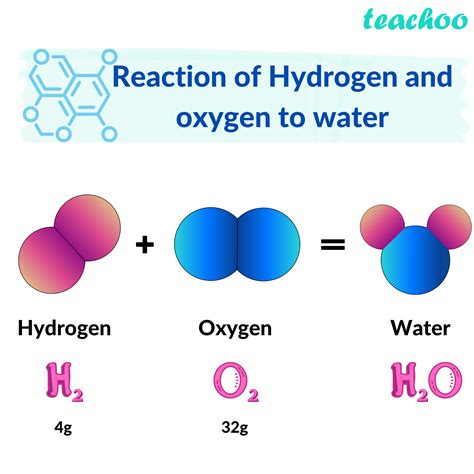
Table of Contents
Water is Always a Product in What Type of Reaction? Understanding Synthesis Reactions
Water, the elixir of life, plays a crucial role in countless chemical reactions. But have you ever stopped to consider the specific types of reactions where water is always a product? The answer lies in understanding the fundamental principles of chemical reactions and focusing on a particular category: synthesis reactions, also known as combination reactions.
Understanding Synthesis Reactions: Combining to Create
A synthesis reaction, at its core, is a chemical process where two or more reactants combine to form a single, more complex product. This process often involves the formation of new chemical bonds between the reacting species. The general formula for a synthesis reaction can be represented as:
A + B → AB
Where A and B are the reactants, and AB represents the single product formed from their combination. The arrow signifies the direction of the reaction. Importantly, the number and type of atoms remain the same on both sides of the equation, obeying the law of conservation of mass.
Water as a Product: The Key Players
While water can be a reactant in certain reactions (like hydrolysis), it's consistently a product in a specific subset of synthesis reactions involving acids and bases. This is where the concept of neutralization comes into play.
Neutralization Reactions: Acids and Bases Unite
Neutralization reactions are a prime example of synthesis reactions where water is invariably produced. These reactions occur when an acid reacts with a base. The defining characteristic of an acid is its ability to donate a proton (H⁺ ion), while a base accepts a proton. The reaction between an acid and a base leads to the formation of water and a salt.
The general equation for a neutralization reaction is:
Acid + Base → Salt + Water
For instance, consider the classic example of hydrochloric acid (HCl) reacting with sodium hydroxide (NaOH):
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
Here, hydrochloric acid, a strong acid, donates a proton to sodium hydroxide, a strong base. The result is the formation of sodium chloride (table salt) and water. The water molecule is formed from the combination of the proton from the acid (H⁺) and the hydroxide ion (OH⁻) from the base. This process effectively neutralizes the acidic and basic properties of the reactants.
Beyond Strong Acids and Bases: Weak Acids and Bases
The neutralization reaction and subsequent water formation aren't limited to strong acids and bases. Weak acids and bases also undergo similar reactions, although the extent of the reaction might vary. For example, consider the reaction between acetic acid (CH₃COOH), a weak acid found in vinegar, and sodium hydroxide:
CH₃COOH(aq) + NaOH(aq) → CH₃COONa(aq) + H₂O(l)
Even though acetic acid is a weak acid, it still donates a proton to sodium hydroxide, resulting in the formation of water and sodium acetate, the salt.
Other Reactions Producing Water: Combustion and Certain Redox Reactions
While neutralization reactions are the most straightforward examples where water is consistently a product, it also appears in other reaction types, though not always as a primary or sole product.
Combustion Reactions: Burning and Water Formation
Combustion reactions, involving the rapid reaction of a substance with an oxidant (usually oxygen), often produce water as a byproduct, particularly when the reactant contains hydrogen. The classic example is the combustion of hydrocarbons (compounds containing carbon and hydrogen). For instance, the complete combustion of methane (CH₄), the main component of natural gas, yields carbon dioxide and water:
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g)
In this reaction, the hydrogen atoms from methane combine with oxygen to form water molecules. The complete combustion of other hydrocarbons follows a similar pattern, always resulting in water as a product alongside carbon dioxide. Incomplete combustion, however, may produce other products like carbon monoxide instead of carbon dioxide.
Redox Reactions: Oxidation and Reduction with Water Formation
Redox (reduction-oxidation) reactions involve the transfer of electrons between reactants. While not all redox reactions produce water, some specific reactions involving hydrogen or oxygen can lead to water formation. For example, the reaction between hydrogen gas and oxygen gas to form water is a classic redox reaction:
2H₂(g) + O₂(g) → 2H₂O(l)
In this reaction, hydrogen is oxidized (loses electrons), and oxygen is reduced (gains electrons), forming water as the product. The reaction is highly exothermic, releasing significant heat energy.
The Importance of Water Formation in Various Reactions
The formation of water in these reactions isn't merely a coincidence; it's often a crucial aspect of the overall process.
Energy Considerations: Exothermic Reactions and Water Formation
Many reactions where water is a product are exothermic, meaning they release heat energy. The formation of the strong O-H bonds in water contributes significantly to the overall energy released in these reactions. This energy release has implications in various applications, from generating power to driving chemical processes.
Importance in Biological Systems: Water's Role in Life
In biological systems, the formation and breakdown of water are fundamental processes. Metabolic reactions, such as cellular respiration and photosynthesis, involve water formation or consumption, playing a critical role in maintaining the balance of life. The properties of water, such as its polarity and ability to act as a solvent, also contribute significantly to the functioning of biological systems.
Conclusion: Water as a Marker of Specific Reaction Types
In summary, while water can participate in various reactions, it's consistently a product in neutralization reactions, where acids and bases react to form salts and water. It's also frequently a product in combustion reactions and some redox reactions, especially those involving hydrogen and oxygen. Understanding these reaction types and the role of water in them provides a deeper understanding of fundamental chemical processes and their significance in both the natural world and human applications. The consistent appearance of water in these reactions highlights its pivotal role in chemistry and beyond. This knowledge is essential for chemists, biologists, and anyone seeking to comprehend the intricate world of chemical transformations.
Latest Posts
Latest Posts
-
22 5 Out Of 30 As A Percentage
Apr 01, 2025
-
How Do You Write 2 As A Decimal
Apr 01, 2025
-
How Many Inches Are In 1 1 2
Apr 01, 2025
-
What Percent Of 600 Is 3
Apr 01, 2025
-
How Tall Is 24 Inches In Feet
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Water Is Always A Product In What Type Of Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
