What Are The Horizontal Rows On The Periodic Table Called
Kalali
Mar 09, 2025 · 6 min read
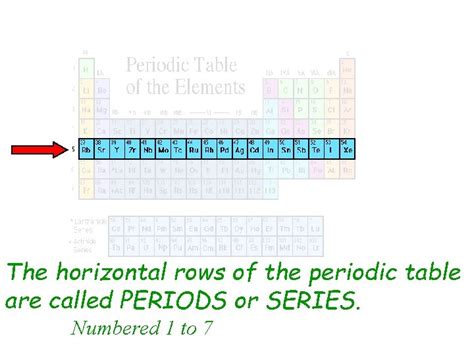
Table of Contents
What Are the Horizontal Rows on the Periodic Table Called? A Deep Dive into Periods
The periodic table, that iconic chart adorning countless science classrooms, is more than just a colorful arrangement of elements. It's a meticulously organized system reflecting fundamental chemical and physical properties. One of the key organizational features is the horizontal rows, known as periods. Understanding periods is crucial to grasping the underlying principles of the periodic table and predicting element behavior. This comprehensive article delves deep into the nature of periods, exploring their significance, the trends they exhibit, and their implications in chemistry.
Understanding Periods: A Foundation of the Periodic Table
The horizontal rows of the periodic table are called periods. Each period represents a principal energy level or shell in an atom. As you move across a period from left to right, the number of electrons in the outermost shell, also known as the valence shell, increases. This progressive filling of the valence shell significantly impacts an element's properties, leading to observable trends in reactivity, atomic size, and ionization energy.
The number of periods directly corresponds to the number of electron shells that can be filled. Currently, there are seven periods on the periodic table, reflecting the seven principal energy levels that can accommodate electrons. Each period begins with the filling of a new electron shell, leading to a systematic increase in the atom's size and a shift in its chemical behavior.
Period 1: The Simplest Beginning
Period 1 is the shortest period, containing only two elements: hydrogen (H) and helium (He). These elements have only one electron shell, with hydrogen possessing one electron and helium possessing two. The filling of this shell marks the completion of the first period. The contrasting properties of hydrogen (highly reactive) and helium (inert) already highlight the impact of electron shell configuration on chemical behavior.
Period 2 and 3: Expanding the Shell
Period 2 and Period 3 each contain eight elements. These periods involve the filling of the second and third electron shells, respectively. These shells can accommodate more electrons, leading to a greater diversity of element properties. The elements in periods 2 and 3 showcase a clear progression in properties, transitioning from highly reactive metals on the left to less reactive nonmetals on the right. This transition highlights the crucial role of valence electrons in determining an element's reactivity.
Periods 4 and 5: Introducing d-Block Elements
Periods 4 and 5 are significantly longer than periods 2 and 3, accommodating 18 elements each. This increase in length is due to the introduction of the d-block elements, also known as transition metals. The d-block elements involve filling of the d orbitals, which are lower in energy than the subsequent p orbitals. This filling pattern leads to a unique set of properties for transition metals, including variable oxidation states, complex ion formation, and catalytic activity. The presence of these elements enriches the chemical landscape significantly.
Periods 6 and 7: f-Block Elements and the Actinides
Periods 6 and 7 are the longest periods, containing 32 elements each. This expansion is due to the addition of the f-block elements, the lanthanides (period 6) and actinides (period 7). The f-block elements involve filling of the f orbitals, adding further complexity to electron configuration and resulting in a unique range of chemical and physical properties. Many f-block elements are radioactive, with practical applications in medicine, energy production, and scientific research.
Periodic Trends Across Periods: A Systematic Variation
As you move across a period from left to right, several important periodic trends emerge, reflecting the gradual increase in the number of protons and electrons. These trends are central to understanding the chemical and physical behavior of the elements.
Atomic Radius: A Decreasing Trend
The atomic radius, the distance from the nucleus to the outermost electron, generally decreases across a period. This is because the increasing nuclear charge pulls the electrons closer to the nucleus, effectively shrinking the atom's size. The added protons outweigh the shielding effect of added electrons in the same energy level.
Ionization Energy: An Increasing Trend
Ionization energy is the energy required to remove an electron from a neutral atom. Ionization energy generally increases across a period due to the increasing nuclear charge, making it more difficult to remove an electron. The stronger attraction between the nucleus and electrons increases the energy needed for ionization.
Electronegativity: An Increasing Trend
Electronegativity measures an atom's ability to attract electrons in a chemical bond. Electronegativity generally increases across a period due to the increasing nuclear charge, making atoms more attractive to shared electrons. Highly electronegative elements strongly attract electrons towards themselves within a molecule.
Electron Affinity: A Complex Trend
Electron affinity refers to the energy change when an atom gains an electron. While there's a general trend toward more negative electron affinities across a period (indicating greater energy release upon electron gain), exceptions exist due to the specific electronic configurations of certain elements. Electron affinity, unlike the other trends, is not always straightforward and requires careful consideration of electronic configurations.
Beyond Trends: The Importance of Valence Electrons
While the periodic trends discussed are valuable, it's essential to remember the paramount role of valence electrons. These are the electrons in the outermost shell, directly involved in chemical bonding. The number of valence electrons dictates an element's reactivity and the types of bonds it can form. Elements in the same group (vertical columns) have the same number of valence electrons and often exhibit similar chemical behavior, highlighting the importance of this electron configuration.
For example, elements in Group 1 (alkali metals) all have one valence electron, making them highly reactive and readily losing this electron to form +1 ions. Similarly, Group 18 elements (noble gases) have a full valence shell (eight electrons, except for helium with two), making them extremely stable and unreactive.
Applications of Periodicity: Predicting Properties and Behavior
Understanding periods and their associated trends allows for the prediction of an element's properties and its likely chemical behavior. This predictive power is immensely valuable in various fields:
-
Material Science: Designing new materials with specific properties often involves selecting elements based on their positions within the periodic table and their anticipated behavior.
-
Drug Design: Understanding the reactivity and electronic properties of elements is crucial in designing pharmaceuticals. Manipulating the electronic structure of molecules can influence their interactions with biological systems.
-
Catalysis: Transition metals, located in the d-block, are often used as catalysts in chemical reactions due to their variable oxidation states and ability to form complexes. Understanding periodic trends aids in catalyst selection and optimization.
-
Nuclear Chemistry: The periodic table is essential in understanding the behavior of radioactive elements. The placement of elements within the table can provide insight into their stability and decay patterns.
Conclusion: Periods – A Key to Unlocking Chemical Understanding
The horizontal rows of the periodic table, or periods, are far more than just a convenient organizational feature. They represent a fundamental principle in the organization of matter, reflecting the filling of electron shells and the resulting periodic trends in atomic and chemical properties. Understanding periods, their length, and the trends they exhibit is crucial to grasping the behavior of elements, predicting their properties, and leveraging this knowledge across various scientific and technological disciplines. From the simplest elements in Period 1 to the complex f-block elements in Periods 6 and 7, the concept of periods is a cornerstone of modern chemistry and a powerful tool for understanding the world around us. Further exploration of the periodic table and its inherent organization will continue to reveal new insights and applications in the years to come.
Latest Posts
Latest Posts
-
How Many Times Does 3 Go Into 30
Jul 13, 2025
-
In What Episode Of Bleach Does Ichigo Ask Orihime Out
Jul 13, 2025
-
How Much Is 4 Oz Chocolate Chips
Jul 13, 2025
-
How Many Times Does 9 Go Into 70
Jul 13, 2025
-
4 Pics 1 Word Cheat 8 Letters
Jul 13, 2025
Related Post
Thank you for visiting our website which covers about What Are The Horizontal Rows On The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.