What Are The Inner Transition Metals
Kalali
Apr 01, 2025 · 6 min read
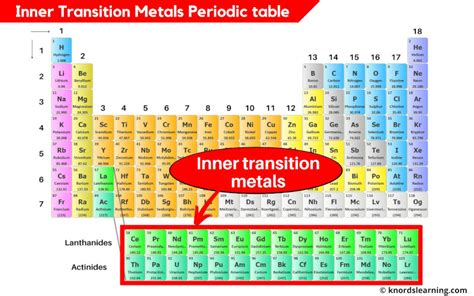
Table of Contents
What are the Inner Transition Metals? A Deep Dive into the f-block Elements
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. While many are familiar with the alkali metals, alkaline earth metals, and transition metals, a less understood group exists: the inner transition metals. These elements, also known as f-block elements, occupy a unique position and possess distinct characteristics that set them apart. This article will delve deep into the world of inner transition metals, exploring their properties, uses, and the fascinating science behind their behavior.
Understanding the Periodic Table Structure and the f-block
Before diving into the specifics of inner transition metals, it's crucial to understand their location within the periodic table's broader structure. The table arranges elements based on increasing atomic number, reflecting the number of protons in their nuclei. Elements are further categorized into groups (columns) and periods (rows). Groups share similar chemical properties due to the same number of valence electrons, while periods represent the filling of electron shells.
The transition metals, occupying the d-block, are characterized by their partially filled d-orbitals. However, nestled below the main body of the periodic table are two rows of elements – the lanthanides (rare earth elements) and the actinides. These elements, with their partially filled f-orbitals, constitute the inner transition metals. Their placement below the main body reflects the late filling of the 4f and 5f orbitals, a consequence of the complex interplay of electron-electron interactions and orbital energies.
The Lanthanides: The First Inner Transition Series
The lanthanides, also known as the rare earth elements, encompass elements 57 (Lanthanum) to 71 (Lutetium). Despite the name "rare earth," many of these elements are not particularly rare in the Earth's crust, though they are often dispersed and challenging to extract in pure form. This difficulty in isolation contributes to their historical designation as "rare."
Properties of Lanthanides:
- Similar Chemical Properties: A defining characteristic of lanthanides is their remarkably similar chemical properties. This similarity stems from the fact that their chemical reactions primarily involve the three electrons in their outermost shell (6s and 5d), while the filling of the 4f orbitals has a comparatively smaller influence on their reactivity. This makes their separation and purification a significant challenge.
- Variable Oxidation States: While +3 is the most common oxidation state for lanthanides, some can exhibit +2 or +4 oxidation states, leading to a diverse range of chemical compounds.
- Paramagnetism: Many lanthanides possess unpaired electrons in their 4f orbitals, making them paramagnetic – they are attracted to magnetic fields.
- Color: Many lanthanide compounds exhibit vibrant colors, a consequence of the electronic transitions within their f-orbitals. This property makes them valuable in various applications, including pigments and lasers.
- Catalysis: Certain lanthanides and their compounds exhibit catalytic activity, making them essential components in various industrial processes.
Uses of Lanthanides:
The applications of lanthanides are diverse and growing, ranging from everyday technologies to advanced materials science:
- Magnets: Neodymium magnets (NdFeB), incorporating neodymium, are exceptionally strong permanent magnets, crucial in numerous applications, including electric motors, wind turbines, and hard disk drives.
- Lighting: Lanthanide compounds are widely used in fluorescent lamps and other lighting applications due to their ability to emit specific wavelengths of light when excited.
- Catalysis: Lanthanides are crucial catalysts in petroleum refining, cracking processes, and other industrial chemical reactions.
- Ceramics: Lanthanide oxides are added to ceramics to enhance their strength, hardness, and other properties.
- Medical Applications: Certain lanthanides are used in medical imaging techniques, such as magnetic resonance imaging (MRI).
The Actinides: The Second Inner Transition Series
The actinides, elements 89 (Actinium) to 103 (Lawrencium), form the second inner transition series. Unlike the lanthanides, most actinides are radioactive and have relatively short half-lives. This radioactivity significantly impacts their properties and applications.
Properties of Actinides:
- Radioactivity: Radioactivity is the most striking characteristic of actinides. Their instability leads to the emission of alpha, beta, or gamma radiation. This radiation poses health risks, necessitating careful handling and storage.
- Variable Oxidation States: Actinides exhibit a wider range of oxidation states than lanthanides, further complicating their chemistry. This is a consequence of the close energy levels of the 5f, 6d, and 7s orbitals.
- Metallic Properties: Like lanthanides, actinides are metals, though their reactivity and other metallic properties can vary considerably due to their radioactivity.
- Chemical Reactivity: Many actinides are highly reactive, particularly with oxygen and moisture.
- Nuclear Properties: The nuclear properties of actinides are of great importance in nuclear energy and weapons technology.
Uses of Actinides:
The applications of actinides are primarily concentrated in nuclear technology:
- Nuclear Fuel: Uranium and Plutonium are crucial elements in nuclear reactors, serving as nuclear fuel to generate electricity.
- Nuclear Weapons: Plutonium's fissile properties make it a key component of nuclear weapons.
- Nuclear Medicine: Certain actinides, despite their radioactivity, find limited use in nuclear medicine for targeted cancer therapy.
- Research: Actinides are extensively studied for their unique nuclear properties and potential applications in various areas of research.
Separating Lanthanides and Actinides: A Challenging Task
The remarkably similar chemical properties of lanthanides make their separation a challenging process. Historically, fractional crystallization was employed, a laborious and time-consuming method. Modern techniques utilize ion-exchange chromatography and solvent extraction, which offer greater efficiency and purity.
Separating actinides presents similar, if not greater, challenges. Their radioactivity adds another layer of complexity, requiring specialized equipment and safety procedures. Methods similar to those used for lanthanides are employed, but the process is significantly more intricate and demanding.
Environmental Impact and Safety Concerns
The production and use of both lanthanides and actinides raise environmental and safety concerns:
- Mining and Processing: The mining and processing of these elements can have significant environmental impacts, including habitat destruction and the release of pollutants.
- Radioactivity: The radioactivity of actinides poses a significant health risk, necessitating careful handling and disposal to prevent contamination.
- Toxicity: While not all lanthanides are highly toxic, some can be harmful if ingested or inhaled. Actinides pose a greater toxic hazard due to their radioactivity.
Ongoing Research and Future Applications
Research on inner transition metals continues to advance, exploring new applications and addressing environmental and safety concerns. This includes:
- Development of new materials: Researchers are investigating the potential of lanthanides and actinides in creating novel materials with unique properties for various applications, including advanced electronics, energy storage, and catalysis.
- Improved separation techniques: Efforts are underway to develop more efficient and environmentally friendly methods for separating and purifying these elements.
- Radioactive waste management: Safe and sustainable management of radioactive waste generated from the use of actinides is a critical area of research.
Conclusion: The Unique World of Inner Transition Metals
The inner transition metals, encompassing the lanthanides and actinides, occupy a unique niche in the periodic table, possessing distinctive properties that have led to a vast array of applications, ranging from everyday technologies to advanced nuclear technologies. While their separation and handling pose significant challenges, ongoing research promises to unlock even greater potential for these fascinating elements in the years to come. Understanding their properties and applications is crucial for navigating the complexities of modern materials science, nuclear technology, and environmental stewardship. From the vibrant colors of lanthanide compounds to the potent energy of actinide isotopes, the inner transition metals offer a rich and ever-evolving area of scientific exploration.
Latest Posts
Latest Posts
-
How Many Gallons Is 10 Cups
Apr 02, 2025
-
What Is 62 Degrees Celsius In Fahrenheit
Apr 02, 2025
-
Is Usa In The Northern Hemisphere
Apr 02, 2025
-
How Many Glasses Is 32 Oz Of Water
Apr 02, 2025
-
How Many Inches Is 183 Cm
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Are The Inner Transition Metals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
