What Compound Directly Provides Energy For Cellular Work
Kalali
Mar 12, 2025 · 6 min read
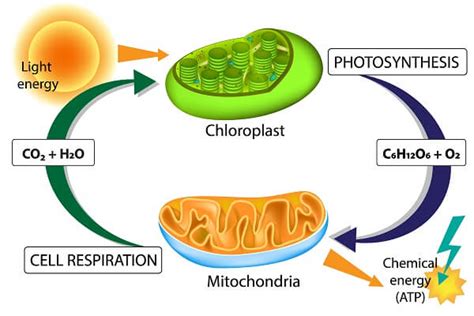
Table of Contents
What Compound Directly Provides Energy for Cellular Work?
The fundamental question driving cellular biology is: what fuels the intricate machinery of life? While we often hear about glucose and calories, the true answer lies in a much smaller, more reactive molecule: adenosine triphosphate (ATP). This remarkable compound is the direct energy currency of the cell, powering virtually every process imaginable, from muscle contraction to protein synthesis to nerve impulse transmission. Understanding ATP's role is crucial to grasping the complexities of cellular metabolism and the maintenance of life itself.
The Role of ATP: The Universal Energy Currency
ATP is a nucleoside triphosphate, meaning it consists of a nitrogenous base (adenine), a five-carbon sugar (ribose), and three phosphate groups. It's the presence of these high-energy phosphate bonds that make ATP so critical. The bonds between the phosphate groups are high-energy because of the electrostatic repulsion between the negatively charged phosphate groups. Hydrolysis, the breaking of these bonds, releases a significant amount of free energy that the cell can harness for work.
ATP Hydrolysis: The Energy Release Mechanism
The process of ATP hydrolysis is the key to understanding ATP's energy-providing role. When a phosphate group is cleaved from ATP, it forms adenosine diphosphate (ADP) and an inorganic phosphate (Pi). This reaction releases approximately 7.3 kcal/mol of free energy under standard conditions. This energy is not released as heat; instead, it's coupled to other endergonic (energy-requiring) reactions within the cell, driving them forward. Think of it like a rechargeable battery: ATP is the charged battery, and ADP is the discharged one.
Coupling ATP Hydrolysis to Cellular Work
The energy released during ATP hydrolysis isn't directly used to power cellular processes. Instead, it's used to create temporary, high-energy intermediates or conformational changes in proteins. This process is known as energy coupling. For example:
- Muscle Contraction: ATP hydrolysis provides the energy needed for the myosin heads to bind to actin filaments, causing muscle fibers to shorten and generate force.
- Active Transport: Many transport proteins utilize ATP hydrolysis to move molecules across cell membranes against their concentration gradients. The sodium-potassium pump is a prime example.
- Protein Synthesis: The formation of peptide bonds during protein synthesis requires energy derived from ATP hydrolysis.
- Nerve Impulse Transmission: The propagation of nerve impulses depends on the movement of ions across neuronal membranes, a process powered by ATP hydrolysis.
- DNA Replication and Repair: The unwinding of DNA and the synthesis of new DNA strands necessitate ATP-driven enzymatic reactions.
How is ATP Synthesized? The Cellular Power Plants
While ATP is the direct energy source, the cell continuously needs to replenish its ATP supply. This crucial process occurs primarily through three main pathways:
1. Substrate-Level Phosphorylation: A Quick Energy Boost
This is the simplest method of ATP synthesis. It involves the direct transfer of a phosphate group from a substrate molecule to ADP, forming ATP. This process occurs in glycolysis and the citric acid cycle (Krebs cycle), generating a relatively small amount of ATP directly. While efficient for immediate energy needs, it's not a major ATP producer compared to oxidative phosphorylation.
2. Oxidative Phosphorylation: The Powerhouse of ATP Production
This is the most significant ATP production pathway, responsible for the vast majority of ATP generated in aerobic organisms. It takes place in the mitochondria, the cell's powerhouses, and involves two crucial stages:
- Electron Transport Chain (ETC): Electrons are passed down a series of protein complexes embedded in the inner mitochondrial membrane. This electron flow creates a proton gradient across the membrane.
- Chemiosmosis: The proton gradient established by the ETC drives protons back across the membrane through ATP synthase, an enzyme that uses this proton flow to synthesize ATP from ADP and Pi. This process is also called oxidative phosphorylation because it requires oxygen as the final electron acceptor.
The ETC and chemiosmosis are incredibly efficient, generating a large amount of ATP per molecule of glucose oxidized. This process is the primary source of energy for most of the body's functions.
3. Photophosphorylation: Harnessing Light Energy
In photosynthetic organisms like plants and algae, light energy is used to generate ATP. This process, known as photophosphorylation, shares similarities with oxidative phosphorylation, involving electron transport and chemiosmosis. However, instead of an electron donor like NADH, light energy excites electrons in chlorophyll, initiating the electron transport chain. This process produces ATP and NADPH, which are used to drive the synthesis of carbohydrates during the Calvin cycle.
Other Energy-Carrying Molecules: Supporting Roles
While ATP is the primary energy currency, other molecules play supporting roles in energy transfer within the cell. These include:
- Creatine Phosphate: A high-energy phosphate compound found in muscle tissue. It can rapidly donate a phosphate group to ADP to regenerate ATP during intense muscular activity, providing a quick burst of energy.
- Guanosine Triphosphate (GTP): Similar to ATP, GTP can be hydrolyzed to release energy. It's involved in various metabolic pathways, including protein synthesis and signal transduction.
- NADH and FADH2: These electron carriers transport high-energy electrons from glycolysis and the citric acid cycle to the electron transport chain, contributing to ATP synthesis during oxidative phosphorylation.
The Importance of Metabolic Regulation
Cellular energy production is tightly regulated to meet the cell's varying energy demands. This regulation involves several mechanisms, including:
- Feedback Inhibition: High ATP levels inhibit enzymes involved in ATP production, slowing down the process when sufficient energy is available. Conversely, low ATP levels stimulate these enzymes.
- Hormonal Control: Hormones like insulin and glucagon play a crucial role in regulating blood glucose levels and, consequently, cellular energy metabolism.
- Allosteric Regulation: Allosteric enzymes involved in glycolysis and the citric acid cycle can be activated or inhibited by binding of small molecules, influencing the rate of ATP production.
Conclusion: ATP – The Cell's Indispensable Energy Source
In summary, adenosine triphosphate (ATP) is the fundamental molecule that directly provides energy for nearly all cellular work. Its high-energy phosphate bonds release energy upon hydrolysis, driving a vast array of cellular processes. The continuous replenishment of ATP through substrate-level phosphorylation, oxidative phosphorylation, and photophosphorylation ensures the cell has a constant supply of energy to maintain life's intricate functions. Understanding the intricacies of ATP metabolism is key to understanding the fundamental processes that drive life itself. Furthermore, disruptions in ATP production can lead to a variety of diseases and cellular dysfunction, highlighting the crucial role this small but mighty molecule plays in maintaining health and well-being. Further research into the regulation and optimization of ATP production remains a significant area of ongoing investigation in biomedical research.
Latest Posts
Latest Posts
-
Who Did Alan Jackson Have An Affair With
Jul 04, 2025
-
How Much Is 500 Grams Of Ground Beef
Jul 04, 2025
-
1 Pound Pasta Is How Many Cups
Jul 04, 2025
-
How Many Cans Of Soda Are In A 2 Liter
Jul 04, 2025
-
How Do U Say Of In Spanish
Jul 04, 2025
Related Post
Thank you for visiting our website which covers about What Compound Directly Provides Energy For Cellular Work . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.