What Does The Atomic Number Represent
Kalali
Mar 21, 2025 · 6 min read
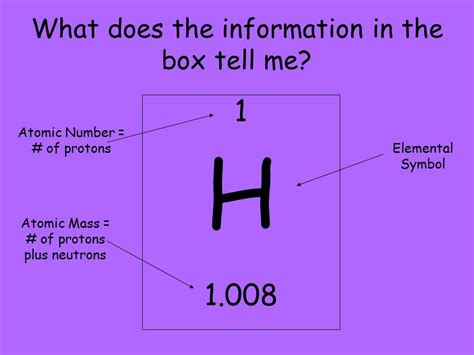
Table of Contents
What Does the Atomic Number Represent? Unlocking the Secrets of the Elements
The periodic table, a cornerstone of chemistry, organizes elements based on their properties. But at the heart of this organization lies a fundamental number: the atomic number. Understanding what the atomic number represents is key to grasping the nature of matter itself. This number isn't just a label; it unlocks a wealth of information about an atom's identity, behavior, and place within the universe of chemical elements.
The Atomic Number: A Definition
Simply put, the atomic number of an element represents the number of protons found in the nucleus of each atom of that element. This is a crucial piece of information because:
-
It defines the element: Each element possesses a unique atomic number. No two elements share the same number of protons. This makes the atomic number the definitive identifier for an element, far more fundamental than its physical properties which can vary under different conditions. For example, water can exist as ice, liquid, or steam, but its constituent atoms—hydrogen and oxygen—retain their unique atomic numbers regardless of the water's phase.
-
It dictates chemical properties: The number of protons directly influences the number of electrons an atom possesses in its neutral state (equal to the number of protons). These electrons determine how the atom interacts with other atoms, forming chemical bonds and determining the element's reactivity and overall chemical behavior. This is because electrons are responsible for participating in chemical reactions.
-
It determines position on the periodic table: The periodic table is arranged in order of increasing atomic number, providing a systematic framework for understanding the relationships between different elements. Elements with similar atomic numbers often share similar chemical properties, leading to the table's arrangement into groups and periods. This organization reveals trends and patterns in elemental characteristics.
Delving Deeper: Protons, Neutrons, and Isotopes
To fully appreciate the significance of the atomic number, we must consider the other subatomic particles within the atom's nucleus: protons and neutrons.
-
Protons: Positively charged particles that contribute to the atom's mass and its positive nuclear charge. As mentioned, the number of protons defines the atomic number.
-
Neutrons: Neutral particles (no charge) that also contribute to the atom's mass. Unlike protons, the number of neutrons in an atom can vary without changing the element's identity.
This variation in neutron number leads to the concept of isotopes. Isotopes are atoms of the same element (same atomic number) that have different numbers of neutrons. For example, carbon-12 (¹²C) has 6 protons and 6 neutrons, while carbon-14 (¹⁴C) has 6 protons and 8 neutrons. Both are carbon, identified by their atomic number of 6, but they differ in their mass number (total number of protons and neutrons). Isotopes can have different properties, including radioactive decay.
Mass Number and Atomic Mass
The mass number is the total number of protons and neutrons in an atom's nucleus. It is an integer value and is denoted by a superscript to the left of the element's symbol (e.g., ¹²C). The atomic mass (or atomic weight), however, is the weighted average of the masses of all the naturally occurring isotopes of an element. This is not a whole number because it reflects the abundance of each isotope in nature. For example, the atomic mass of carbon is approximately 12.011 amu (atomic mass units), reflecting the mixture of ¹²C, ¹³C, and trace amounts of other carbon isotopes.
The Atomic Number and Chemical Reactivity
The atomic number directly impacts an element's chemical reactivity because it dictates the number of electrons in the atom's electron shells. These electrons are arranged in specific energy levels or orbitals. Atoms strive to achieve a stable electron configuration, often by gaining, losing, or sharing electrons with other atoms. This process leads to the formation of chemical bonds and the creation of molecules and compounds.
Valence Electrons and Chemical Bonding
The valence electrons are the electrons in the outermost energy level of an atom. They are the electrons most involved in chemical bonding. The number of valence electrons is closely related to the atomic number and determines an element's reactivity. Elements in the same group (vertical column) on the periodic table have the same number of valence electrons and, consequently, tend to exhibit similar chemical behavior.
For example, the alkali metals (Group 1) all have one valence electron, making them highly reactive as they readily lose this electron to achieve a stable electron configuration. In contrast, the noble gases (Group 18) have a full outer electron shell (eight valence electrons, except for helium with two), making them extremely unreactive and stable.
The Atomic Number and Nuclear Chemistry
The atomic number plays a vital role in understanding nuclear reactions. Nuclear reactions involve changes in the nucleus of an atom, including changes in the number of protons and neutrons.
-
Radioactive Decay: Radioactive isotopes undergo spontaneous decay, emitting particles or energy to achieve a more stable nuclear configuration. The atomic number can change during decay processes such as alpha decay (loss of two protons and two neutrons), beta decay (conversion of a neutron to a proton or vice versa), and gamma decay (emission of high-energy photons).
-
Nuclear Fission: The splitting of a heavy atomic nucleus into two lighter nuclei, often accompanied by the release of a substantial amount of energy. The atomic numbers of the resulting nuclei are different from the original nucleus.
-
Nuclear Fusion: The combining of two light atomic nuclei to form a heavier nucleus, also releasing significant energy. The atomic number of the resulting nucleus is the sum of the atomic numbers of the original nuclei.
The Atomic Number: A Foundation of Modern Science
The concept of the atomic number, seemingly simple in its definition, is fundamental to a wide range of scientific disciplines. Its significance extends beyond basic chemistry and physics:
-
Materials Science: Understanding the atomic numbers of constituent elements allows researchers to design materials with specific properties, from high-strength alloys to superconductors.
-
Nuclear Medicine: Radioactive isotopes, characterized by specific atomic numbers and decay properties, are used in medical imaging and cancer treatment.
-
Astrophysics: Analyzing the light emitted from stars and other celestial objects allows scientists to determine their elemental composition based on the unique spectral lines associated with different atomic numbers.
-
Geochemistry: The atomic numbers of elements provide valuable insights into the formation and evolution of the Earth and other planets.
Conclusion: Beyond a Simple Number
The atomic number, representing the number of protons in an atom's nucleus, is far more than a simple numerical identifier. It is a fundamental property that dictates an element's identity, chemical behavior, position on the periodic table, and involvement in various chemical and nuclear processes. Its importance permeates numerous scientific fields, highlighting its crucial role in our understanding of the universe and the matter that constitutes it. By grasping the significance of the atomic number, we unlock a deeper appreciation for the intricate structure and remarkable properties of the elements that shape our world.
Latest Posts
Latest Posts
-
How Many Acres Is A Square Mile
Mar 28, 2025
-
15 Out Of 50 As A Percentage
Mar 28, 2025
-
How Many Inches In 8 Cm
Mar 28, 2025
-
How To Find Slope Of Vector
Mar 28, 2025
-
How Tall Is 163cm In Feet
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Does The Atomic Number Represent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
