What Happens To Mass During Alpha Decay
Kalali
Mar 16, 2025 · 6 min read
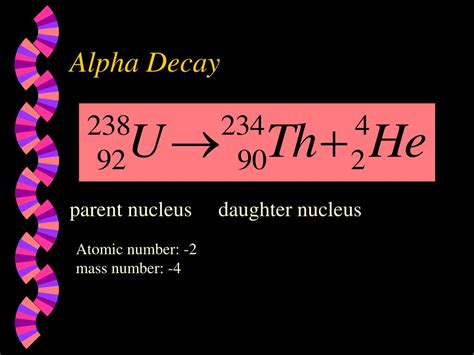
Table of Contents
- What Happens To Mass During Alpha Decay
- Table of Contents
- What Happens to Mass During Alpha Decay?
- Understanding Alpha Decay
- Mass Defect and Binding Energy
- Mass Change Calculation
- Energy Released and Kinetic Energy
- Conservation Laws in Alpha Decay
- Factors Affecting Alpha Decay
- Applications and Significance
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
What Happens to Mass During Alpha Decay?
Alpha decay, a fundamental process in nuclear physics, involves the emission of an alpha particle from a radioactive nucleus. This seemingly simple event has profound implications for the mass of the atom involved. Understanding what happens to mass during this decay is crucial for grasping the underlying principles of nuclear stability and radioactive decay. This article will delve deep into the intricacies of alpha decay, explaining the mass changes involved, the energy released, and the conservation laws that govern this process.
Understanding Alpha Decay
Alpha decay is a type of radioactive decay where an unstable atomic nucleus emits an alpha particle, effectively transforming into a different nuclide. An alpha particle is identical to a helium-4 nucleus, consisting of two protons and two neutrons. This means it carries a +2 charge and a mass number of 4. The emission of this particle significantly alters the parent nucleus, reducing its atomic number by 2 and its mass number by 4.
The Decay Equation:
A typical alpha decay equation can be represented as:
²⁰⁸₈₂Pb → ²⁰⁴₈₀Hg + ⁴₂He
In this example, Lead-208 (²⁰⁸₈₂Pb) undergoes alpha decay, emitting an alpha particle (⁴₂He) and transforming into Mercury-204 (²⁰⁴₈₀Hg). The superscripts represent the mass numbers (total number of protons and neutrons), and the subscripts represent the atomic numbers (number of protons). Note that the sum of the mass numbers and the sum of the atomic numbers are conserved on both sides of the equation.
Mass Defect and Binding Energy
The key to understanding mass changes during alpha decay lies in the concept of mass defect and binding energy. The mass of a nucleus is always less than the sum of the masses of its individual constituent protons and neutrons. This difference in mass, the mass defect, is converted into energy according to Einstein's famous equation, E=mc², where 'E' is energy, 'm' is mass, and 'c' is the speed of light. This energy is known as the binding energy and holds the nucleus together.
The binding energy per nucleon (the number of protons and neutrons in a nucleus) is a measure of the stability of the nucleus. Nuclei with higher binding energy per nucleon are more stable. Alpha decay occurs because the daughter nucleus (the nucleus remaining after alpha decay) and the alpha particle together have a higher total binding energy than the parent nucleus. This difference in binding energy is released as kinetic energy of the alpha particle and the recoiling daughter nucleus.
Mass Change Calculation
Let's analyze the mass change during alpha decay more quantitatively. We can use the mass of the parent nucleus, the mass of the daughter nucleus, and the mass of the alpha particle to determine the mass defect during the decay process. These masses can be found in nuclear physics data tables, often expressed in atomic mass units (amu).
For our example (²⁰⁸₈₂Pb → ²⁰⁴₈₀Hg + ⁴₂He), let's assume the following masses (these are approximate values and can vary slightly depending on the source):
- Mass of ²⁰⁸₈₂Pb: 207.9766 amu
- Mass of ²⁰⁴₈₀Hg: 203.9735 amu
- Mass of ⁴₂He: 4.0026 amu
The sum of the masses of the daughter nucleus and the alpha particle is: 203.9735 amu + 4.0026 amu = 207.9761 amu
The difference between the mass of the parent nucleus and the sum of the masses of the daughter nucleus and the alpha particle is:
207.9766 amu - 207.9761 amu = 0.0005 amu
This small mass difference (0.0005 amu) represents the mass that is converted into energy during the alpha decay process. While this seems insignificant, when converted using E=mc², it results in a significant amount of kinetic energy carried away by the alpha particle and the recoiling daughter nucleus. This energy is what makes alpha decay detectable and measurable.
Energy Released and Kinetic Energy
The energy released during alpha decay can be calculated using Einstein's mass-energy equivalence formula: E=mc². We need to convert the mass difference (0.0005 amu) into kilograms and then use the speed of light (c = 3 x 10⁸ m/s). The conversion factor from amu to kg is approximately 1.66 x 10⁻²⁷ kg/amu.
Therefore, the energy released is approximately:
E = (0.0005 amu) * (1.66 x 10⁻²⁷ kg/amu) * (3 x 10⁸ m/s)² ≈ 7.47 x 10⁻¹⁴ Joules
This energy is primarily manifested as kinetic energy of the alpha particle and the recoiling daughter nucleus. The alpha particle carries away a larger proportion of this kinetic energy due to its smaller mass compared to the daughter nucleus. The exact distribution of kinetic energy depends on the momentum conservation principle.
Conservation Laws in Alpha Decay
Several fundamental conservation laws govern alpha decay:
- Conservation of Mass-Energy: The total mass-energy of the system remains constant. The mass lost is converted into kinetic energy.
- Conservation of Charge: The total charge of the system remains constant. The +2 charge of the alpha particle is balanced by the decrease in atomic number of the parent nucleus.
- Conservation of Momentum: The total momentum of the system remains constant. The momentum of the alpha particle and the recoiling daughter nucleus are equal and opposite.
- Conservation of Nucleon Number: The total number of nucleons (protons and neutrons) remains constant. The loss of four nucleons in the alpha particle is compensated for by the decrease in mass number of the parent nucleus.
Factors Affecting Alpha Decay
Several factors influence the rate and characteristics of alpha decay:
- Nuclear Size: Larger nuclei are more likely to undergo alpha decay because of the increased Coulomb repulsion between protons.
- Nuclear Structure: The specific arrangement of protons and neutrons within the nucleus affects the stability and the likelihood of alpha decay. Nuclei with "magic numbers" of protons or neutrons (2, 8, 20, 28, 50, 82, 126) are generally more stable and less prone to alpha decay.
- Binding Energy: As discussed earlier, the difference in binding energy between the parent and daughter nuclei determines the energy released during alpha decay.
Applications and Significance
Understanding alpha decay is vital in various fields:
- Nuclear Physics: It provides insights into the structure and stability of atomic nuclei.
- Radioactive Dating: The predictable rate of alpha decay is used in radiometric dating techniques to determine the age of geological formations and artifacts.
- Radiation Detection: Alpha particles can be detected using various methods, allowing for the measurement of radioactivity.
- Nuclear Medicine: Alpha-emitting isotopes are being explored in targeted alpha therapy, a promising cancer treatment modality.
Conclusion
Alpha decay represents a fascinating process where the mass of a nucleus is subtly but significantly altered. This mass defect, though small in magnitude, is converted into a substantial amount of kinetic energy according to Einstein's mass-energy equivalence. The conservation laws that govern alpha decay ensure a balanced and predictable outcome, making it a cornerstone concept in nuclear physics. Its understanding is essential for advancements in various fields, from nuclear energy and medicine to geological dating and the ongoing quest to unravel the secrets of the atom. The intricate interplay of mass, energy, and nuclear forces in alpha decay continues to inspire research and provide a deeper comprehension of the fundamental workings of the universe.
Latest Posts
Latest Posts
-
How Many Cups Of Water Is In 2 Quarts
Mar 17, 2025
-
How Many 2 Quarts In A Cup
Mar 17, 2025
-
2 3 4 Inches To Mm
Mar 17, 2025
-
How Much Is 1 1 4 Cup Of Water
Mar 17, 2025
-
How Tall Is 76 Inches In Feet
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Happens To Mass During Alpha Decay . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
