What Holds The Sides Of Dna Ladder Together
Kalali
Mar 15, 2025 · 6 min read
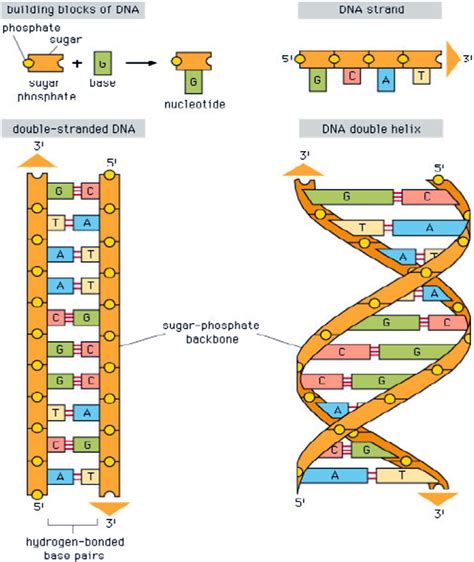
Table of Contents
What Holds the Sides of the DNA Ladder Together? A Deep Dive into Hydrogen Bonds and Beyond
The iconic double helix structure of DNA, often depicted as a twisted ladder, is a marvel of biological engineering. But what exactly holds this ladder together? While the simplified answer points to hydrogen bonds, the complete picture is far richer and involves a complex interplay of forces that ensure the stability and functionality of this crucial molecule. This article delves deep into the mechanics of DNA's structure, exploring the key roles of hydrogen bonds, base stacking interactions, and the surrounding aqueous environment.
The Foundation: Hydrogen Bonds Between Base Pairs
The rungs of the DNA ladder are formed by nitrogenous bases, specifically adenine (A), guanine (G), cytosine (C), and thymine (T). These bases pair up in a specific manner: adenine always pairs with thymine (A-T), and guanine always pairs with cytosine (G-C). This specificity is crucial for accurate DNA replication and transcription.
The key to this specific pairing lies in hydrogen bonds. These are relatively weak bonds formed between a hydrogen atom covalently bonded to a highly electronegative atom (like oxygen or nitrogen) and another electronegative atom. In DNA, these bonds occur between the bases:
-
A-T base pair: Two hydrogen bonds are formed between adenine and thymine. One bond forms between the nitrogen of adenine and the oxygen of thymine, and the other forms between the amino group of adenine and the carbonyl group of thymine.
-
G-C base pair: Three hydrogen bonds are formed between guanine and cytosine. These bonds involve the amino and carbonyl groups of both bases, leading to a stronger interaction compared to the A-T base pair.
The number of hydrogen bonds influences the strength of the base pairing. The G-C base pair, with its three hydrogen bonds, is generally considered stronger than the A-T base pair with its two hydrogen bonds. This difference in strength has implications for DNA melting temperature (the temperature at which the double helix separates) and the stability of DNA under various conditions.
The Specificity of Base Pairing: A Closer Look
The precise geometry of the bases is critical for hydrogen bond formation. The arrangement of hydrogen bond donor and acceptor atoms in adenine perfectly complements that in thymine, and similarly for guanine and cytosine. Any other pairing would lead to steric clashes and prevent the formation of stable hydrogen bonds. This precise matching ensures the fidelity of DNA replication and the accurate transmission of genetic information.
Beyond Hydrogen Bonds: The Importance of Base Stacking
While hydrogen bonds are crucial for the specificity of base pairing, they only account for a fraction of the overall stability of the DNA double helix. A significant contribution comes from base stacking interactions, also known as π-π stacking. These interactions arise from the aromatic nature of the nitrogenous bases.
The planar aromatic rings of the bases are stacked on top of each other in the DNA helix. This stacking leads to van der Waals interactions and hydrophobic effects that contribute significantly to the overall stability of the DNA structure. These forces are weaker than hydrogen bonds individually, but their cumulative effect is substantial. The hydrophobic effect, in particular, arises from the tendency of the nonpolar bases to cluster together and minimize their contact with the surrounding water molecules.
The Role of the Hydrophobic Effect
The DNA double helix exists in an aqueous environment. The hydrophobic nature of the bases drives them inwards, away from the surrounding water, promoting base stacking. This hydrophobic effect contributes significantly to the stability of the double helix and influences the DNA's conformation and interactions with other molecules.
The DNA Backbone: Sugar-Phosphate Interactions
The sides of the DNA ladder, also known as the backbone, consist of alternating sugar (deoxyribose) and phosphate groups. These components are linked together by phosphodiester bonds, strong covalent bonds that form the robust structural framework of the DNA molecule. These bonds are essential for maintaining the overall integrity of the double helix and preventing its spontaneous disassembly.
The sugar-phosphate backbone is negatively charged due to the phosphate groups. This negative charge influences the DNA's interactions with proteins and other molecules, playing a significant role in processes like DNA packaging and replication. The negatively charged backbone also contributes to the stability of the double helix by repelling each other, preventing the collapse of the structure.
Environmental Factors Affecting DNA Stability
The stability of the DNA double helix isn't solely determined by its intrinsic structural features. External factors such as temperature, pH, and ionic strength also play important roles.
-
Temperature: Increasing the temperature provides the energy needed to break the hydrogen bonds between base pairs, leading to DNA denaturation or melting. The melting temperature (Tm) is the temperature at which half of the DNA molecules are denatured. G-C rich regions have higher Tm values due to the increased number of hydrogen bonds.
-
pH: Extreme pH values can alter the ionization state of the bases, affecting hydrogen bond formation and the overall stability of the DNA structure.
-
Ionic strength: The presence of ions in the solution can shield the negative charges of the phosphate backbone, reducing electrostatic repulsion and potentially affecting the stability of the DNA helix.
Proteins and DNA Interactions: A Dynamic System
DNA does not exist in isolation. It interacts with a wide range of proteins that regulate its replication, transcription, repair, and packaging. These proteins often recognize specific DNA sequences and bind to them through various interactions, including hydrogen bonds, hydrophobic interactions, and electrostatic interactions. These interactions are crucial for the proper functioning of the genome.
Conclusion: A Multifaceted Interaction
In conclusion, the stability of the DNA double helix is a result of a complex interplay of various forces. While hydrogen bonds are essential for the specific base pairing that determines the genetic code, base stacking interactions and the hydrophobic effect contribute significantly to the overall stability of the structure. The negatively charged sugar-phosphate backbone and its interactions with the surrounding environment further influence DNA's stability and its interactions with other molecules. Understanding these diverse interactions is fundamental to comprehending the structure, function, and dynamics of this vital molecule. The intricate balance of these forces ensures that the genetic blueprint of life is faithfully preserved and utilized. Further research continues to unravel the finer details of these interactions, revealing the remarkable complexity and elegance of DNA's molecular architecture. The interplay of hydrogen bonds, base stacking, and the surrounding environment creates a remarkably stable yet dynamic structure capable of performing its vital functions within the cell. This detailed understanding of DNA’s stability is crucial not only for basic biological research but also for advancements in fields like medicine and biotechnology, where manipulating and understanding DNA is paramount.
Latest Posts
Latest Posts
-
What Separates A Salamander From A Turtle
Mar 15, 2025
-
Is The Atlantic Ocean Warmer Than The Pacific
Mar 15, 2025
-
Que Porcentaje Es 20 De 27
Mar 15, 2025
-
What Is 0 2 As A Percentage
Mar 15, 2025
-
How Many Combinations Of Phone Numbers Are There
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Holds The Sides Of Dna Ladder Together . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
