What Holds The Sides Of The Dna Ladder Together
Kalali
Mar 19, 2025 · 6 min read
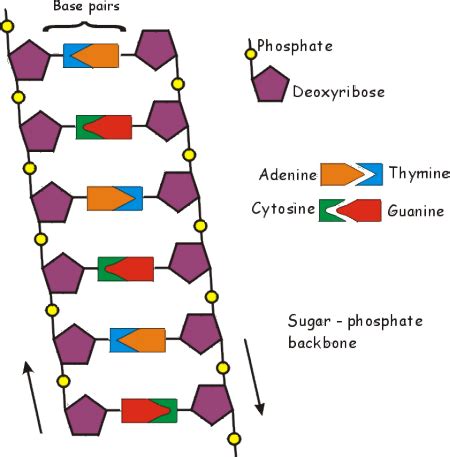
Table of Contents
What Holds the Sides of the DNA Ladder Together? Unlocking the Secrets of the Double Helix
The iconic image of DNA – a twisted ladder, or double helix – is instantly recognizable. But what actually holds this elegant structure together? The answer is far more intricate and fascinating than a simple "glue" might suggest. Understanding the forces and interactions that maintain the integrity of the DNA ladder is crucial to comprehending the fundamental mechanisms of life itself. This article delves deep into the molecular mechanisms responsible for holding the DNA double helix together, exploring the key players and the subtle nuances of their interactions.
The Backbone: The Sugar-Phosphate Structure
The sides of the DNA ladder aren't held together by a single type of bond; instead, they're formed by a continuous backbone composed of alternating sugar and phosphate groups. This backbone is fundamental to the structural integrity of the entire molecule.
The Sugar: Deoxyribose
Each sugar molecule is deoxyribose, a five-carbon sugar specifically lacking one oxygen atom compared to ribose, the sugar found in RNA. This seemingly small difference has significant implications for DNA's stability. The absence of the oxygen contributes to DNA's greater resistance to hydrolysis (breakdown by water), a critical factor for the long-term storage of genetic information. The deoxyribose molecules are linked together in a specific way, creating the backbone's structural rigidity.
The Phosphate: Connecting the Sugars
The phosphate groups are the crucial linking agents, connecting the 3' carbon of one deoxyribose to the 5' carbon of the next. This phosphodiester bond, a strong covalent bond, is responsible for the continuous and robust nature of the sugar-phosphate backbone. The negative charge of the phosphate groups also plays a critical role, influencing the DNA's interactions with its environment, particularly its interactions with positively charged ions and proteins.
The Rungs: Hydrogen Bonds and Base Pairing
While the backbone provides the structural framework, the rungs of the DNA ladder are formed by the interaction of nitrogenous bases. These bases are the "letters" of the genetic code, and their specific pairing is the key to DNA replication and protein synthesis.
The Nitrogenous Bases: Adenine, Guanine, Cytosine, and Thymine
Four types of nitrogenous bases are found in DNA: adenine (A), guanine (G), cytosine (C), and thymine (T). These bases are planar, aromatic molecules that stack neatly within the double helix. Their specific arrangement is crucial for the complementary base pairing that holds the two strands together.
Base Pairing: A-T and G-C
Adenine (A) always pairs with thymine (T), and guanine (G) always pairs with cytosine (C). This pairing is dictated by hydrogen bonding. A and T form two hydrogen bonds, while G and C form three hydrogen bonds. These hydrogen bonds are relatively weak individually, but the cumulative effect of many hydrogen bonds along the DNA molecule provides substantial stability. The specific geometry of the bases allows for optimal hydrogen bond formation, maximizing the stability of the base pairs. The specificity of base pairing is crucial for faithful DNA replication and the accurate transmission of genetic information.
The Importance of Hydrogen Bonds
The use of hydrogen bonds in base pairing is a masterstroke of evolutionary design. These bonds are strong enough to hold the two DNA strands together under normal cellular conditions but weak enough to be broken during DNA replication and transcription. This allows for the controlled unwinding and rewinding of the DNA molecule, facilitating the essential processes of genetic information processing.
Beyond Hydrogen Bonds: Other Interactions Contributing to DNA Stability
While hydrogen bonds are the primary force responsible for base pairing, several other interactions contribute significantly to the overall stability of the DNA double helix.
Hydrophobic Interactions: Base Stacking
The bases themselves are relatively hydrophobic (water-repelling), tending to cluster together in the interior of the double helix. This stacking of bases contributes significantly to the stability of the DNA molecule, minimizing contact between the bases and the surrounding water molecules. The base stacking interactions are influenced by the specific sequence of bases, and they are particularly important for the helical structure.
Van der Waals Forces: Weak but Ubiquitous
Van der Waals forces are weak, short-range attractions between molecules. Although individually weak, the cumulative effect of numerous van der Waals forces along the DNA molecule adds to its overall stability. These forces contribute to the precise arrangement of the bases and the overall compact structure of the double helix.
Ionic Interactions: The Role of Counterions
The negatively charged phosphate groups in the backbone attract positively charged ions (counterions), such as magnesium ions (Mg²⁺). These ions neutralize some of the negative charge, reducing the electrostatic repulsion between the phosphate groups and further stabilizing the DNA structure. These counterions effectively shield the negative charges, preventing the two strands from repelling each other too strongly.
The Double Helix: A Dynamic Structure
It's important to remember that the DNA double helix isn't a static, rigid structure. It's a dynamic molecule, constantly undergoing subtle conformational changes in response to its environment and interacting proteins. The flexibility of the DNA molecule is crucial for its biological functions, allowing for processes such as bending, unwinding, and supercoiling.
DNA Supercoiling: Compact Packaging
In cells, DNA is often supercoiled, meaning that the double helix itself is further twisted and coiled to compact the molecule. This supercoiling plays a crucial role in DNA packaging, allowing the vast amount of genetic information to be efficiently stored within the limited space of a cell nucleus. The supercoiling also affects the accessibility of the DNA to proteins involved in replication and transcription.
DNA Bending and Flexibility
DNA can also bend and flex, allowing it to interact with various proteins that are involved in gene regulation, DNA replication, and DNA repair. These conformational changes are essential for the dynamic nature of the cellular processes that involve DNA.
Conclusion: A Symphony of Interactions
The stability of the DNA double helix isn't due to a single, all-powerful force, but rather a complex interplay of several interactions. The strong covalent phosphodiester bonds in the backbone provide the fundamental structural framework, while the hydrogen bonds between base pairs ensure the specificity of base pairing and the overall stability of the double helix. In addition, hydrophobic interactions, van der Waals forces, and ionic interactions all contribute significantly to the stability of the DNA molecule. This delicate balance of interactions allows for the remarkable flexibility and dynamic nature of DNA, crucial for its role in the processes of life. Understanding these interactions is key to understanding the very essence of heredity and the mechanisms that maintain the integrity of our genetic blueprint. The DNA double helix is a testament to the elegance and sophistication of molecular design, a structure optimized for the robust storage and controlled manipulation of the information that defines life itself.
Latest Posts
Latest Posts
-
2 To The Power Of 20
Mar 20, 2025
-
How Many Feet Is 47 In
Mar 20, 2025
-
What Is 41 Inches In Feet
Mar 20, 2025
-
What Is The 30 Of 100
Mar 20, 2025
-
How Many Cups Is 5 Quarts Of Water
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Holds The Sides Of The Dna Ladder Together . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
