What Is A Horizontal Row Called On The Periodic Table
Kalali
Mar 11, 2025 · 7 min read
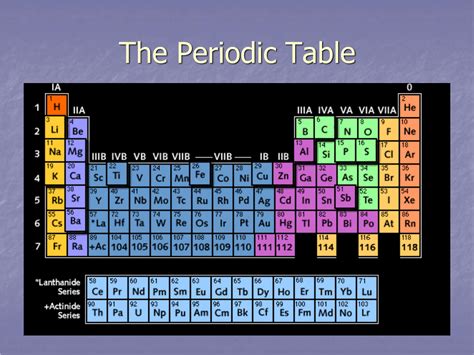
Table of Contents
What is a Horizontal Row Called on the Periodic Table? Understanding Periods and Their Significance
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. Understanding its structure is crucial for comprehending the behavior of matter. One fundamental aspect of this structure is the arrangement of elements into horizontal rows. But what exactly is a horizontal row called on the periodic table? The answer is a period. This article will delve into the definition of a period, explore its significance in understanding elemental properties, and discuss how the periodic trends within a period relate to atomic structure.
Defining a Period: Horizontal Organization of Elements
A period in the periodic table is a horizontal row that arranges elements in increasing order of atomic number. Each period represents a principal energy level (or shell) that is being filled with electrons. The number of elements in each period varies, reflecting the different ways electrons fill subshells within those energy levels. This variation is dictated by the quantum mechanical model of the atom and the specific rules governing electron configuration.
The Significance of Period Number
The period number corresponds directly to the highest principal quantum number (n) of the electrons in their ground state. For instance, elements in Period 1 (Hydrogen and Helium) have electrons only in the n=1 energy level. Elements in Period 2 (Lithium to Neon) have electrons in the n=1 and n=2 energy levels, with the outermost electrons residing in the n=2 level. This simple correlation between period number and energy level allows us to quickly infer the electron shell structure of an element just by knowing its location on the table.
Trends in Properties Across a Period: A Closer Look
As we move across a period from left to right, we observe systematic changes, or trends, in various physical and chemical properties. These trends are directly linked to the increasing number of protons in the nucleus and the consequent changes in electron configuration. Understanding these trends is fundamental to predicting the reactivity and behavior of elements.
1. Atomic Radius: A Decreasing Trend
Atomic radius, the distance from the nucleus to the outermost electron, generally decreases across a period. As we add protons to the nucleus, the positive charge increases, pulling the electrons closer to the center. While additional electrons are being added to the same energy level, the increased nuclear charge outweighs the effect of electron-electron repulsion, leading to a smaller atomic radius.
2. Ionization Energy: An Increasing Trend
Ionization energy is the energy required to remove an electron from a gaseous atom. This energy generally increases across a period. The stronger attractive force from the increasing nuclear charge makes it harder to remove an electron, hence the higher ionization energy. Exceptions can arise due to electron configuration changes, particularly when moving past half-filled or completely filled subshells.
3. Electronegativity: A Generally Increasing Trend
Electronegativity, a measure of an atom's ability to attract electrons in a chemical bond, generally increases across a period. Similar to ionization energy, the increasing nuclear charge enhances an atom's pull on shared electrons in a covalent bond, resulting in higher electronegativity. This trend is particularly significant in determining the nature of chemical bonds (ionic or covalent) formed between elements.
4. Electron Affinity: A Complex Trend
Electron affinity, the energy change associated with adding an electron to a neutral atom, shows a more complex trend across a period. While a general increase is observed, there are exceptions stemming from electron configurations and electron-electron repulsions. Adding an electron to a stable configuration (like a full subshell) is less favorable energetically than adding to an incomplete subshell.
5. Metallic Character: A Decreasing Trend
Metallic character, representing the tendency of an element to lose electrons and form positive ions, generally decreases across a period. As electronegativity increases, the tendency to hold onto electrons strengthens, reducing the metallic nature. This is reflected in the transition from highly reactive metals on the left to nonmetals and eventually noble gases on the right.
The Role of Electron Configuration in Periodic Trends
The observed periodic trends are a direct consequence of the changes in electron configuration across a period. As we move from left to right, electrons are added successively to the same principal energy level, but within different subshells. This affects the shielding effect of inner electrons and the overall attraction between the nucleus and the valence electrons, which are the outermost electrons and primarily responsible for chemical bonding and reactivity.
Periods and the Organization of the Periodic Table: A Deeper Dive
The organization of the periodic table into periods is not merely a convenient arrangement; it reflects fundamental principles of atomic structure and quantum mechanics. The number of elements in each period is directly related to the number of orbitals available at each principal energy level.
Period 1: The Smallest Period
Period 1 contains only two elements, Hydrogen (H) and Helium (He). This is because the first energy level (n=1) has only one subshell, the 1s subshell, which can hold a maximum of two electrons.
Period 2 and 3: The Second and Third Short Periods
Periods 2 and 3 each contain eight elements. This is because the second and third energy levels have four subshells (2s, 2p, 3s, and 3p), which can accommodate a total of eight electrons in their valence shells.
Periods 4 and 5: The Long Periods
Periods 4 and 5 each contain 18 elements. This increase is due to the filling of the d subshells (3d and 4d respectively), which can hold a maximum of 10 electrons in addition to the s and p subshells already present.
Periods 6 and 7: The Longest Periods
Periods 6 and 7 are the longest periods, each containing 32 elements. The significant increase arises from the filling of the f subshells (4f and 5f respectively), which can hold a maximum of 14 electrons, further increasing the number of elements in these periods.
Understanding the Exceptions and Irregularities
While the periodic trends provide a general framework for understanding the behavior of elements, it is crucial to acknowledge that exceptions exist. Electron-electron repulsions, particularly in partially filled subshells, can influence the observed properties, leading to deviations from the expected trends. Careful examination of electron configurations and their effect on effective nuclear charge helps explain these deviations.
Practical Applications of Understanding Periods
The understanding of periods and their associated trends is not merely an academic exercise. It has significant practical applications in various fields:
- Material Science: Predicting the properties of new materials is crucial for designing advanced materials with specific functionalities. The understanding of periodic trends allows scientists to tailor the properties of materials by selecting elements with desired characteristics.
- Chemical Synthesis: Designing chemical reactions and predicting their outcomes often rely on understanding the reactivity of elements. Knowing the position of an element in the periodic table and its associated periodic trends can greatly aid in optimizing reaction conditions and predicting product formation.
- Environmental Chemistry: Understanding the behavior of elements in the environment requires knowledge of their chemical properties. The periodic table helps us understand the mobility, toxicity, and bioavailability of different elements in various environmental settings.
- Biochemistry: The periodic table is central to understanding the role of trace elements in biological systems. The unique properties of elements like iron, zinc, and copper are crucial for enzyme activity and overall biological function.
Conclusion: The Importance of Periods in Chemistry
In conclusion, the horizontal rows in the periodic table, known as periods, are far more than a simple organizational tool. They are a powerful representation of the underlying principles of atomic structure and electron configuration. Understanding the trends in atomic properties across periods is fundamental to comprehending the chemical behavior of elements and predicting their reactivity. This knowledge has widespread applications in numerous scientific disciplines, emphasizing the critical role periods play in our understanding of the chemical world. By mastering the concept of periods, we gain a deeper insight into the intricate organization and predictable patterns that govern the elements and their interactions.
Latest Posts
Latest Posts
-
How Many Grams Of Sugar In A Pound
Jul 12, 2025
-
7am To 11am Is How Many Hours
Jul 12, 2025
-
If Your 35 What Year Was You Born
Jul 12, 2025
-
How Many Cups Is 1 Pound Of Cheese
Jul 12, 2025
-
30 X 30 Is How Many Square Feet
Jul 12, 2025
Related Post
Thank you for visiting our website which covers about What Is A Horizontal Row Called On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.