What Is A Positive Ion Called
Kalali
Mar 30, 2025 · 5 min read
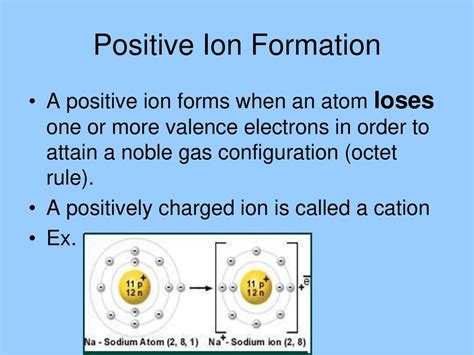
Table of Contents
What is a Positive Ion Called? A Deep Dive into Cations and Their Significance
A positive ion, also known as a cation, is an atom or molecule that has lost one or more electrons. This loss of electrons results in a net positive charge, as the number of protons (positively charged particles) now exceeds the number of electrons (negatively charged particles). Understanding cations is crucial in various fields, from chemistry and physics to biology and medicine. This article delves into the intricacies of positive ions, exploring their formation, properties, nomenclature, and significance across different scientific disciplines.
The Formation of Cations: A Balancing Act
The formation of a cation typically involves the loss of valence electrons. Valence electrons are the outermost electrons in an atom, and they are the ones most readily involved in chemical bonding. Atoms tend to achieve stability by having a full outermost electron shell, often resembling the electron configuration of a noble gas. For many atoms, this stable configuration can be achieved by losing electrons, thus forming a positively charged ion.
Several factors influence the ease with which an atom forms a cation:
- Ionization Energy: This is the energy required to remove an electron from a neutral atom. Elements with low ionization energies tend to readily form cations. Alkali metals (Group 1), for example, have very low ionization energies and readily lose one electron to achieve a stable noble gas configuration.
- Electrostatic Forces: The positive charge of the nucleus attracts electrons. The stronger this attraction, the more difficult it is to remove an electron. This is why elements with high nuclear charge tend to have higher ionization energies and are less likely to form cations.
- Electron Shielding: Inner electrons shield the outer electrons from the full positive charge of the nucleus. Elements with many inner electrons experience less effective nuclear charge, making it easier to remove outer electrons and form cations.
The process of cation formation is often associated with exothermic reactions, meaning they release energy. This energy release contributes to the stability of the resulting cation.
Examples of Cation Formation:
- Sodium (Na): Sodium, an alkali metal, readily loses one valence electron to form a sodium cation (Na⁺), achieving the stable electron configuration of neon.
- Magnesium (Mg): Magnesium loses two valence electrons to become a magnesium cation (Mg²⁺), also achieving a noble gas configuration (like neon).
- Aluminum (Al): Aluminum loses three valence electrons to form an aluminum cation (Al³⁺), resembling the neon configuration.
- Transition Metals: Transition metals exhibit variable oxidation states, meaning they can lose different numbers of electrons to form cations with varying charges (e.g., Fe²⁺ and Fe³⁺ for iron).
Properties of Cations: Size, Charge, and Reactivity
Cations possess distinct properties that differentiate them from their neutral atom counterparts:
- Smaller Size: When an atom loses electrons, it loses an electron shell, resulting in a smaller ionic radius compared to the neutral atom. This is because the remaining electrons are pulled closer to the nucleus by the increased effective nuclear charge.
- Positive Charge: The defining characteristic of a cation is its positive charge, resulting from the loss of electrons. The magnitude of the charge depends on the number of electrons lost.
- Reactivity: Cations are often highly reactive, particularly those with higher charges. Their positive charge attracts negatively charged ions (anions) and other electron-rich species, leading to the formation of ionic compounds.
Nomenclature of Cations: Naming the Positively Charged Ions
Naming cations follows specific rules depending on the element:
- Monatomic Cations (single-atom cations): These are simply named after the element with the word "ion" added. For example, Na⁺ is called a sodium ion, Mg²⁺ is a magnesium ion, and Al³⁺ is an aluminum ion. The charge is indicated using Roman numerals in parentheses for transition metals that can form cations with multiple charges (e.g., Iron(II) ion for Fe²⁺ and Iron(III) ion for Fe³⁺).
- Polyatomic Cations (cations containing multiple atoms): These are named according to specific rules based on the constituent atoms. For example, NH₄⁺ is called ammonium ion, and H₃O⁺ is called hydronium ion.
Significance of Cations Across Disciplines
Cations play a vital role in numerous scientific fields:
1. Chemistry:
- Ionic Bonding: Cations form the basis of ionic bonding, where electrostatic attraction between cations and anions leads to the formation of stable ionic compounds like NaCl (sodium chloride).
- Chemical Reactions: Cations participate in a wide array of chemical reactions, acting as reactants, intermediates, or products.
- Electrochemistry: Cations are crucial in electrochemical processes like electrolysis and batteries, where their movement generates electrical current.
2. Biology:
- Electrolyte Balance: Cations like Na⁺, K⁺, Ca²⁺, and Mg²⁺ are essential electrolytes in biological systems, maintaining osmotic balance, nerve impulse transmission, and muscle contraction.
- Enzyme Activity: Many enzymes require specific cations as cofactors for their activity.
- Mineral Nutrition: Plants absorb essential mineral cations like K⁺, Ca²⁺, and Mg²⁺ from the soil for growth and development.
3. Medicine:
- Electrolyte Disorders: Imbalances in cation levels (e.g., hyponatremia, hypokalemia, hypocalcemia) can lead to various health problems.
- Drug Delivery: Certain drugs utilize cationic molecules to facilitate their transport across cell membranes.
- Medical Imaging: Cationic contrast agents are used in medical imaging techniques such as MRI and CT scans to enhance image quality.
4. Materials Science:
- Materials Properties: The presence and arrangement of cations in materials significantly affect their properties like strength, conductivity, and reactivity.
- Catalysis: Cations are often incorporated into catalysts to enhance their activity and selectivity.
- Synthesis of New Materials: Cations are used in the synthesis of a wide range of materials with novel properties.
5. Environmental Science:
- Water Quality: Cation levels in water sources are important indicators of water quality and potential environmental pollution.
- Soil Chemistry: Cation exchange capacity of soils influences nutrient availability to plants.
- Atmospheric Chemistry: Cations play roles in atmospheric processes like acid rain formation.
Conclusion: The Ubiquity of Cations
Positive ions, or cations, are fundamental components of matter and play critical roles in countless natural and technological processes. Their properties, determined by their charge and size, influence their reactivity and interactions with other species. From the basic building blocks of ionic compounds to their essential functions in biological systems and their wide-ranging applications in various technologies, cations are ubiquitous and profoundly important in understanding the world around us. Further research into the behavior and applications of cations will continue to drive advancements in many scientific and technological fields.
Latest Posts
Latest Posts
-
How Many Gallons Is 7 Litres
Apr 01, 2025
-
160 Degrees Celsius Is What In Fahrenheit
Apr 01, 2025
-
62 C Is What In F
Apr 01, 2025
-
Which Type Of Mutation Stops The Translation Of Mrna
Apr 01, 2025
-
500 Mg Is Equal To How Many Grams
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is A Positive Ion Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
