What Is Meant By The Term Saturated When Describing Hydrocarbons
Kalali
Apr 01, 2025 · 6 min read
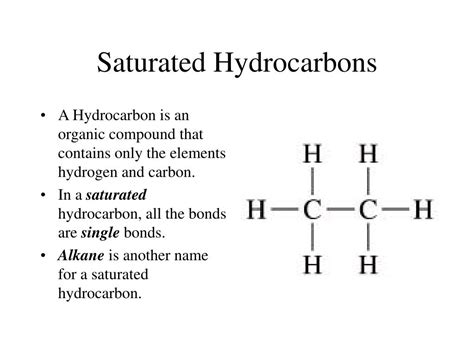
Table of Contents
- What Is Meant By The Term Saturated When Describing Hydrocarbons
- Table of Contents
- What is Meant by the Term Saturated When Describing Hydrocarbons?
- Understanding Saturation: Single Bonds and Stability
- The Contrast: Unsaturated Hydrocarbons
- Alkanes: The Archetypal Saturated Hydrocarbons
- Straight-Chain and Branched-Chain Alkanes
- Properties of Saturated Hydrocarbons
- Physical Properties
- Chemical Properties
- Nomenclature of Saturated Hydrocarbons (IUPAC System)
- Basic Steps for Naming Alkanes:
- Significance of Saturated Hydrocarbons
- Fuels and Energy
- Petrochemicals and Plastics
- Lubricants and Solvents
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
What is Meant by the Term Saturated When Describing Hydrocarbons?
Hydrocarbons are organic compounds consisting solely of hydrogen and carbon atoms. They form the basis of many fuels and are crucial components of countless materials. Within the vast world of hydrocarbons, one crucial distinction lies in their level of saturation. Understanding what "saturated" means in the context of hydrocarbons is fundamental to grasping their properties and applications. This comprehensive guide will delve deep into the meaning of saturated hydrocarbons, exploring their structure, properties, nomenclature, and significance.
Understanding Saturation: Single Bonds and Stability
The term "saturated" in organic chemistry, particularly when discussing hydrocarbons, refers to the maximum number of hydrogen atoms bonded to the carbon atoms. This maximum bonding capacity is achieved when each carbon atom forms four single bonds. These single bonds are essentially pairs of shared electrons, creating a strong and stable connection between the atoms. Crucially, the absence of double or triple bonds is the defining characteristic of a saturated hydrocarbon.
The Contrast: Unsaturated Hydrocarbons
To fully appreciate the meaning of saturated, it's essential to understand its counterpart: unsaturated hydrocarbons. These hydrocarbons contain at least one carbon-carbon double bond (C=C) or triple bond (C≡C). The presence of these multiple bonds alters the molecular structure significantly, impacting their reactivity and properties. Unsaturated hydrocarbons, like alkenes (containing C=C) and alkynes (containing C≡C), possess fewer hydrogen atoms compared to their saturated counterparts with the same number of carbon atoms. This is because the double or triple bond utilizes some of the bonding capacity of the carbons that would otherwise be used to bond with hydrogen.
Alkanes: The Archetypal Saturated Hydrocarbons
Saturated hydrocarbons are also known as alkanes. They form the simplest and most fundamental class of hydrocarbons. Their general formula is C<sub>n</sub>H<sub>2n+2</sub>, where 'n' represents the number of carbon atoms. This formula perfectly encapsulates the concept of saturation; every carbon atom is bonded to the maximum number of hydrogen atoms possible, ensuring no multiple bonds exist within the molecule.
Straight-Chain and Branched-Chain Alkanes
Alkanes can exist as straight-chain structures or branched-chain structures. Straight-chain alkanes, also known as normal alkanes (n-alkanes), have all carbon atoms arranged in a continuous, unbranched chain. Examples include methane (CH₄), ethane (C₂H₆), propane (C₃H₈), and butane (C₄H₁₀).
Branched-chain alkanes possess carbon atoms branching off from the main chain. The presence of branching significantly impacts the physical properties, such as boiling point and melting point. Isomers, which are molecules with the same molecular formula but different structural arrangements, are common among branched-chain alkanes. For example, butane (C₄H₁₀) has two isomers: n-butane (straight chain) and isobutane (branched chain).
Properties of Saturated Hydrocarbons
The saturated nature of alkanes profoundly influences their chemical and physical properties.
Physical Properties
-
Low Melting and Boiling Points: Compared to other types of organic compounds, alkanes have relatively low melting and boiling points. These points increase with the increasing number of carbon atoms due to stronger van der Waals forces. Shorter chain alkanes are gases at room temperature, while longer chains are liquids or solids.
-
Non-Polarity: Alkanes are non-polar molecules due to the small difference in electronegativity between carbon and hydrogen. This non-polar nature results in their insolubility in polar solvents like water but good solubility in non-polar solvents like organic solvents.
-
Low Reactivity: Alkanes are generally unreactive at room temperature. Their stable C-C and C-H single bonds require significant energy to break, making them resistant to many chemical reactions. They are often referred to as "paraffins," which means "little affinity," highlighting their low reactivity.
-
Density: Alkanes are less dense than water, which is why they float on water.
Chemical Properties
-
Combustion: Alkanes readily undergo combustion, reacting with oxygen to produce carbon dioxide, water, and a significant amount of heat. This is the primary reason why alkanes are widely used as fuels. The complete combustion equation for an alkane is: C<sub>n</sub>H<sub>2n+2</sub> + (3n+1)/2 O₂ → nCO₂ + (n+1)H₂O
-
Halogenation: Alkanes can react with halogens (like chlorine or bromine) in a process called halogenation. This reaction typically requires UV light or high temperatures to initiate and involves the substitution of a hydrogen atom with a halogen atom. This reaction can be represented for chlorination as: C<sub>n</sub>H<sub>2n+2</sub> + Cl₂ → C<sub>n</sub>H<sub>2n+1</sub>Cl + HCl
-
Cracking: Long-chain alkanes can be broken down into smaller alkanes and alkenes through a process known as cracking. This process is crucial in the petroleum industry for converting long-chain hydrocarbons into more valuable shorter-chain components for gasoline and other products.
Nomenclature of Saturated Hydrocarbons (IUPAC System)
The International Union of Pure and Applied Chemistry (IUPAC) provides a systematic way to name alkanes. The naming system is based on the number of carbon atoms in the longest continuous chain.
Basic Steps for Naming Alkanes:
-
Identify the longest continuous carbon chain: This determines the base name of the alkane.
-
Number the carbon atoms: Begin numbering from the end that gives the substituents (branches) the lowest possible numbers.
-
Name the substituents: Branched alkyl groups are named using prefixes like methyl (CH₃-), ethyl (C₂H₅-), propyl (C₃H₇-), etc.
-
Combine the names: The names of the substituents are placed alphabetically before the base name, with their positions indicated by numbers. Numbers are separated from words by hyphens, and numbers are separated from each other by commas.
Example: Consider the branched alkane with the formula C₅H₁₂:
!
This compound is named 2-methylbutane. The longest chain has four carbons (butane), and a methyl group is attached to the second carbon atom.
Significance of Saturated Hydrocarbons
Saturated hydrocarbons are ubiquitous in our daily lives and play crucial roles in various industries:
Fuels and Energy
Alkanes are the primary components of natural gas (mostly methane) and petroleum (a complex mixture of alkanes, cycloalkanes, and other hydrocarbons). They are extensively used as fuels for heating, transportation, and electricity generation. Their high energy content upon combustion makes them invaluable energy sources.
Petrochemicals and Plastics
The petrochemical industry uses alkanes as building blocks for the production of countless materials, including plastics, synthetic fibers, solvents, and detergents. Cracking and other refining processes transform alkanes into more useful feedstock chemicals for these manufacturing processes.
Lubricants and Solvents
Certain saturated hydrocarbons with higher molecular weights are used as lubricants, greases, and paraffin waxes. Their non-polar nature makes them suitable for various applications requiring lubrication or solvent properties.
Conclusion
The concept of "saturated" in the context of hydrocarbons is paramount to understanding their properties and applications. The absence of double or triple bonds in saturated hydrocarbons, namely alkanes, leads to their characteristic low reactivity, non-polarity, and specific physical properties. Their significance across various industries, particularly in fuels, petrochemicals, and lubricants, highlights their importance in modern society. Understanding the structure, properties, and nomenclature of saturated hydrocarbons is essential for anyone studying chemistry or working in related fields.
Latest Posts
Latest Posts
-
A Bus Travels 280 Km South
Apr 05, 2025
-
What Is 45 C In Fahrenheit
Apr 05, 2025
-
How Tall Is 86 Inches In Feet
Apr 05, 2025
-
What Are Two Parts Of A Phospholipid
Apr 05, 2025
-
How Many Feet Is 2 5 Metres
Apr 05, 2025
Related Post
Thank you for visiting our website which covers about What Is Meant By The Term Saturated When Describing Hydrocarbons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
