What Is The Boiling Point Of Water In Kelvin
Kalali
Mar 24, 2025 · 5 min read
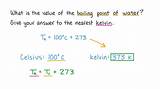
Table of Contents
What is the Boiling Point of Water in Kelvin? A Deep Dive into Temperature Scales and Phase Transitions
The seemingly simple question, "What is the boiling point of water in Kelvin?" opens a door to a fascinating world of temperature scales, phase transitions, and the fundamental properties of matter. While the answer itself is straightforward – 373.15 K – understanding why this is the case requires exploring the concepts behind temperature measurement and the behavior of water molecules. This comprehensive guide delves into the intricacies of this seemingly simple question, providing a robust understanding for both beginners and those seeking a deeper appreciation of thermodynamics.
Understanding Temperature Scales
Before we delve into the boiling point of water specifically, let's establish a firm grasp of the different temperature scales used in scientific and everyday contexts. Three main scales dominate: Celsius (°C), Fahrenheit (°F), and Kelvin (K).
Celsius (°C)
The Celsius scale, also known as the centigrade scale, is widely used globally. It defines 0°C as the freezing point of water and 100°C as its boiling point at standard atmospheric pressure (1 atmosphere or 101.325 kPa). This scale is based on the properties of water, making it convenient for many applications.
Fahrenheit (°F)
The Fahrenheit scale is primarily used in the United States. Its zero point and 100-degree mark are based on arbitrary points, making it less intuitive for scientific purposes. The freezing point of water is 32°F, and the boiling point is 212°F at standard atmospheric pressure.
Kelvin (K)
The Kelvin scale, however, stands apart. It's the absolute temperature scale, meaning its zero point (0 K) represents absolute zero, the theoretically lowest possible temperature. At absolute zero, all molecular motion ceases. This makes the Kelvin scale ideal for scientific applications, as it provides a consistent and fundamental measure of thermal energy. There are no negative values on the Kelvin scale.
The Relationship Between Celsius and Kelvin
The Kelvin scale is directly related to the Celsius scale:
K = °C + 273.15
This simple equation allows for easy conversion between the two scales. For instance, 0°C is equivalent to 273.15 K, and 100°C is 373.15 K. This relationship is crucial for understanding the boiling point of water in Kelvin.
Boiling Point of Water: A Phase Transition
The boiling point of water is the temperature at which liquid water transitions into its gaseous state, water vapor or steam. This transition is a phase change, a process driven by the kinetic energy of water molecules.
Molecular Dynamics and Phase Transitions
At lower temperatures, water molecules are held relatively close together by intermolecular forces (hydrogen bonding). As temperature increases, the kinetic energy of these molecules increases, causing them to move more rapidly and vibrate more intensely. When sufficient energy is gained, the molecules overcome the intermolecular forces and escape the liquid phase, transitioning into the gaseous phase.
Standard Atmospheric Pressure and Boiling Point
It's crucial to note that the boiling point of water is dependent on the ambient pressure. At standard atmospheric pressure (1 atm), water boils at 100°C or 373.15 K. However, at higher altitudes, where atmospheric pressure is lower, the boiling point decreases. This is because the reduced pressure requires less energy for water molecules to escape the liquid phase. Conversely, at higher pressures, the boiling point increases.
Why is the Boiling Point of Water Important?
The boiling point of water is a fundamental constant in numerous scientific and practical applications.
Calibration and Standardization
It serves as a key reference point for calibrating thermometers and other temperature-measuring instruments. The precise boiling point of water at standard pressure ensures accuracy and consistency in scientific measurements.
Cooking and Food Preparation
In culinary arts, the boiling point of water plays a critical role in cooking various dishes. Boiling water is used for cooking pasta, vegetables, and other food items, and understanding its boiling point is essential for achieving optimal results.
Industrial Processes
Many industrial processes rely on the boiling point of water. Steam generation for power plants, sterilization procedures, and various chemical reactions all utilize the properties of water at its boiling point.
Scientific Research
The boiling point of water is essential in various scientific experiments and research, especially in thermodynamics, chemistry, and physics. It serves as a reference point for understanding phase transitions and the behavior of matter under different conditions.
Factors Affecting the Boiling Point of Water
While 373.15 K is the boiling point at standard pressure, several factors can influence this temperature:
Pressure
As previously mentioned, pressure has the most significant impact. Lower pressure leads to a lower boiling point, while higher pressure results in a higher boiling point. This principle is exploited in pressure cookers, where increased pressure allows for higher cooking temperatures, leading to faster cooking times.
Impurities
Dissolved substances in water can slightly alter its boiling point. However, the effect is generally small unless a significant amount of solute is present.
Isotopes
The isotopic composition of water can also subtly affect the boiling point. Water molecules containing heavier isotopes of hydrogen (deuterium) or oxygen have slightly higher boiling points. However, this effect is generally negligible for most applications.
Beyond the Boiling Point: Exploring the Phase Diagram of Water
The boiling point is just one aspect of water's behavior. The phase diagram of water provides a comprehensive representation of its different phases (solid, liquid, gas) under varying conditions of temperature and pressure. The diagram showcases the transitions between these phases, including melting, freezing, boiling, and condensation. Understanding the phase diagram is crucial for comprehending water's behavior in various environments.
Conclusion
The boiling point of water in Kelvin, 373.15 K, is a seemingly simple yet fundamentally important value. It's a cornerstone of numerous scientific disciplines and has practical applications across various fields. Understanding the concept requires a solid grasp of temperature scales, phase transitions, and the influence of external factors on the boiling point. This comprehensive exploration demonstrates that the simple question, "What is the boiling point of water in Kelvin?" leads to a wealth of knowledge about the properties of water and the principles of thermodynamics. This understanding extends far beyond a simple numerical answer, illuminating the fascinating world of physics and chemistry.
Latest Posts
Latest Posts
-
6 Is What Percent Of 50
Mar 26, 2025
-
How Much Is 150 Ml In Ounces
Mar 26, 2025
-
What Is 37 Degrees Fahrenheit In Celsius
Mar 26, 2025
-
How Many Inches Is 43 Centimeters
Mar 26, 2025
-
Cuanto Es Una Yarda En Pies
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Boiling Point Of Water In Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
