What Is The Charge Of Oh
Kalali
Mar 14, 2025 · 5 min read
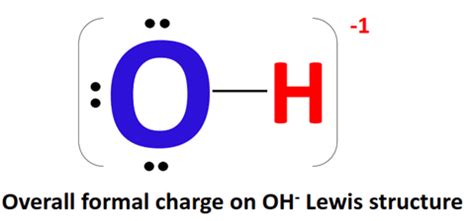
Table of Contents
What is the Charge of OH? Understanding Hydroxide and its Role in Chemistry
The question "What is the charge of OH?" seems simple, but understanding the answer requires delving into the fascinating world of chemistry, specifically the behavior of ions and polyatomic ions. Let's break down the charge of the hydroxide ion (OH⁻) and explore its significance in various chemical contexts.
Understanding Ions and Polyatomic Ions
Before we delve into the specifics of the hydroxide ion, it's crucial to grasp the fundamental concepts of ions and polyatomic ions.
-
Ions: Ions are atoms or molecules that carry an electric charge. This charge arises from an imbalance in the number of protons (positively charged particles) and electrons (negatively charged particles). If an atom loses electrons, it becomes a positively charged ion, called a cation. Conversely, if an atom gains electrons, it becomes a negatively charged ion, called an anion.
-
Polyatomic Ions: Unlike monatomic ions (single atoms with a charge), polyatomic ions are groups of two or more atoms covalently bonded together that carry a net electric charge. These ions act as a single unit in chemical reactions. The hydroxide ion (OH⁻) is a prime example of a polyatomic anion.
The Hydroxide Ion (OH⁻): Structure and Charge
The hydroxide ion, denoted as OH⁻, consists of one oxygen atom and one hydrogen atom covalently bonded together. It carries a negative one charge (-1). This negative charge arises because the oxygen atom in the hydroxide ion has gained an extra electron.
Why the negative charge? Oxygen is highly electronegative, meaning it has a strong tendency to attract electrons. In the OH bond, oxygen attracts the shared electrons more strongly than hydrogen. This unequal sharing of electrons results in a slightly negative charge on the oxygen atom and a slightly positive charge on the hydrogen atom (polar covalent bond). However, the overall charge of the hydroxide ion is -1, owing to the extra electron acquired by the oxygen atom.
Formation of the Hydroxide Ion
The hydroxide ion is typically formed through the dissociation of a base in water. Strong bases, such as sodium hydroxide (NaOH), completely dissociate in water, releasing hydroxide ions:
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
Weak bases, such as ammonia (NH₃), only partially dissociate, resulting in a lower concentration of hydroxide ions in the solution.
The Importance of the Hydroxide Ion in Chemistry
The hydroxide ion plays a crucial role in numerous chemical processes and reactions. Let's explore some key areas:
1. Acidity and Basicity (pH):
The concentration of hydroxide ions (OH⁻) is directly related to the pH of a solution. A high concentration of hydroxide ions indicates a high pH, signifying a basic or alkaline solution. The relationship between hydroxide ion concentration and pH is defined by the pOH scale, which is inversely related to the pH scale.
2. Neutralization Reactions:
Hydroxide ions are central to neutralization reactions, where acids and bases react to form water and a salt. In these reactions, hydroxide ions react with hydronium ions (H₃O⁺) to produce water molecules:
OH⁻(aq) + H₃O⁺(aq) → 2H₂O(l)
3. Precipitation Reactions:
Many metal hydroxides are insoluble in water and will precipitate out of solution when hydroxide ions are added to a solution containing metal cations. This principle is used in various analytical and industrial processes for separating and purifying metals.
For instance, adding sodium hydroxide to a solution containing iron(III) ions (Fe³⁺) will cause the precipitation of iron(III) hydroxide:
Fe³⁺(aq) + 3OH⁻(aq) → Fe(OH)₃(s)
4. Organic Chemistry:
Hydroxide ions play a significant role in many organic reactions. They act as nucleophiles (electron-rich species) that can attack electrophilic carbons (electron-deficient carbons). This is fundamental in reactions like nucleophilic substitution and elimination.
5. Industrial Applications:
The hydroxide ion has numerous industrial applications, including:
- Soap and detergent manufacturing: Hydroxide ions are crucial in the saponification process which converts fats and oils into soaps.
- Wastewater treatment: Hydroxide ions are used to neutralize acidic wastewater and remove heavy metals.
- Pulp and paper industry: Hydroxide ions are used in the pulping process to separate lignin from cellulose fibers.
Differentiating Hydroxide from other Ions:
It's important to differentiate the hydroxide ion from other ions with similar chemical formulas or names. For instance, it's crucial to distinguish OH⁻ from:
-
Hydroxyl group (-OH): This is a functional group found in many organic molecules, such as alcohols and phenols. While it has the same atoms as the hydroxide ion, it's not charged and doesn't carry an overall negative charge. The hydroxyl group is covalently bound within a larger molecule; the hydroxide ion is an independent ion.
-
Superoxide (O₂⁻): This is a different polyatomic ion consisting of two oxygen atoms with a single negative charge.
The Charge of OH in Different Contexts:
While the charge of the hydroxide ion is consistently -1, its role and behavior can vary depending on the chemical environment. The concentration of hydroxide ions, the presence of other ions, and the overall pH of the solution will greatly influence its reactivity and impact.
Conclusion:
The charge of OH is unequivocally -1. This seemingly simple answer opens a door to a complex and multifaceted understanding of the hydroxide ion's crucial role in chemistry. Its influence extends from basic acid-base chemistry and pH determination to more complex areas like precipitation reactions, organic chemistry, and numerous industrial processes. Understanding its charge and behavior is essential for anyone working in chemistry, biochemistry, or related fields. The hydroxide ion is a fundamental building block of our chemical world, participating in countless reactions and shaping the properties of many substances. Its constant negative charge of -1 is the key to understanding its reactivity and its importance in the diverse roles it plays.
Latest Posts
Latest Posts
-
How Does The Monster Try To Gain Control Of Victor
Jul 14, 2025
-
Why Did The Cow Want A Divorce
Jul 14, 2025
-
Lowest Common Denominator For 3 4 5
Jul 14, 2025
-
Can You Be A Professor With A Masters
Jul 14, 2025
-
How Many Bottles Of Water Is 1 Liter
Jul 14, 2025
Related Post
Thank you for visiting our website which covers about What Is The Charge Of Oh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.