What Is The Difference Between A Product And A Reactant
Kalali
Mar 15, 2025 · 7 min read
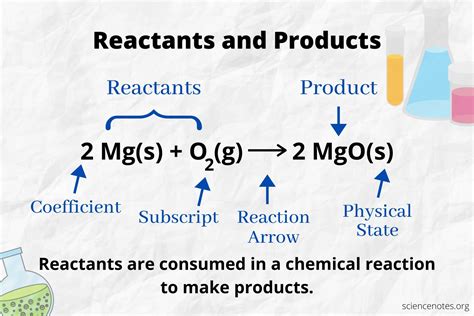
Table of Contents
What's the Difference Between a Product and a Reactant? A Deep Dive into Chemical Reactions
Understanding the difference between products and reactants is fundamental to grasping the essence of chemistry. While seemingly simple, the distinction holds crucial weight in comprehending chemical reactions, predicting outcomes, and designing experiments. This comprehensive guide delves deep into the definitions, characteristics, and practical applications of products and reactants, exploring their roles in various chemical processes. We’ll unpack the concepts with clear examples and address common misconceptions.
Defining Reactants and Products
At the heart of any chemical reaction lies a transformation – the rearrangement of atoms and molecules. Substances undergoing this transformation are called reactants, while the new substances formed are called products. This simple distinction is the cornerstone of chemical stoichiometry and balanced chemical equations.
Reactants: The Starting Materials
Reactants are the initial substances involved in a chemical reaction. They are the ingredients that are consumed during the reaction process, undergoing a change in their chemical composition and structure. Think of them as the "inputs" in a chemical process. They can be elements (like hydrogen or oxygen), compounds (like water or carbon dioxide), or a mixture of both.
Key Characteristics of Reactants:
- Undergo chemical change: Reactants actively participate in the reaction, losing their original properties and forming new ones.
- Consumed during the reaction: The amount of reactants decreases as the reaction proceeds until they are completely used up (or reach equilibrium).
- Appear on the left-hand side of a chemical equation: Chemical equations use an arrow (→) to indicate the direction of the reaction, with reactants listed on the left and products on the right.
- Determine the products formed: The nature and quantities of reactants dictate the type and amount of products generated, governed by the stoichiometric relationships within the balanced equation.
Products: The Result of the Reaction
Products are the substances formed as a result of a chemical reaction. They are the newly created substances with different properties from the reactants. They are the "outputs" of the chemical process. Like reactants, products can be elements or compounds or a mixture of both.
Key Characteristics of Products:
- Formed during the chemical change: Products are created through the rearrangement of atoms and bonds within the reactants.
- Appear on the right-hand side of a chemical equation: They are displayed after the arrow (→) in a chemical equation.
- Possess different properties than reactants: Products typically exhibit distinct physical and chemical properties compared to the initial reactants. This change in properties is evidence of a chemical reaction.
- Their formation is governed by stoichiometry: The amount of product formed is dictated by the amounts of reactants and the stoichiometric ratios outlined in the balanced chemical equation.
Visualizing Reactants and Products: Chemical Equations
Chemical equations serve as shorthand representations of chemical reactions, illustrating the transformation of reactants into products. A correctly balanced chemical equation maintains the law of conservation of mass; the number of atoms of each element remains consistent on both sides of the equation.
Let's consider a simple example: the combustion of methane (CH₄) in oxygen (O₂).
CH₄ + 2O₂ → CO₂ + 2H₂O
In this equation:
- Reactants: Methane (CH₄) and Oxygen (O₂) are the reactants, the starting materials that are consumed in the reaction.
- Products: Carbon dioxide (CO₂) and water (H₂O) are the products, the new substances formed as a result of the reaction. Note the different chemical compositions and properties compared to the reactants.
The equation shows that one molecule of methane reacts with two molecules of oxygen to produce one molecule of carbon dioxide and two molecules of water. This ratio, which maintains mass balance, is crucial in stoichiometric calculations.
Reactants and Products in Different Reaction Types
The concepts of reactants and products apply universally across all types of chemical reactions. Let's examine a few key reaction types:
1. Synthesis Reactions (Combination Reactions)
Synthesis reactions involve the combination of two or more substances to form a more complex substance. The reactants are simpler compounds, and the product is a more complex compound.
Example: The formation of water from hydrogen and oxygen.
2H₂ + O₂ → 2H₂O
Here, hydrogen (H₂) and oxygen (O₂) are the reactants, while water (H₂O) is the product.
2. Decomposition Reactions
Decomposition reactions are the reverse of synthesis reactions. A single compound breaks down into two or more simpler substances. The reactant is a complex compound, and the products are simpler compounds or elements.
Example: The decomposition of calcium carbonate.
CaCO₃ → CaO + CO₂
Calcium carbonate (CaCO₃) is the reactant, while calcium oxide (CaO) and carbon dioxide (CO₂) are the products.
3. Single Displacement Reactions (Substitution Reactions)
Single displacement reactions involve one element replacing another element in a compound. One reactant is an element, and another is a compound. The products consist of a new element and a new compound.
Example: The reaction of zinc with hydrochloric acid.
Zn + 2HCl → ZnCl₂ + H₂
Zinc (Zn) and hydrochloric acid (HCl) are the reactants, while zinc chloride (ZnCl₂) and hydrogen gas (H₂) are the products.
4. Double Displacement Reactions (Metathesis Reactions)
Double displacement reactions involve the exchange of ions between two compounds. The reactants are two compounds, and the products are two new compounds.
Example: The reaction between silver nitrate and sodium chloride.
AgNO₃ + NaCl → AgCl + NaNO₃
Silver nitrate (AgNO₃) and sodium chloride (NaCl) are the reactants, and silver chloride (AgCl) and sodium nitrate (NaNO₃) are the products.
5. Combustion Reactions
Combustion reactions are rapid reactions that produce heat and light, typically involving the reaction of a substance with oxygen. A fuel (often a hydrocarbon) and oxygen are the reactants, while the products typically include carbon dioxide and water, along with energy in the form of heat and light.
Example: The combustion of propane.
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O + Heat + Light
Beyond Simple Reactions: Complex Chemical Processes
The concepts of reactants and products extend beyond simple reactions to encompass complex chemical processes involving multiple steps and intermediate compounds. In these scenarios, the products of one reaction step might serve as reactants in subsequent steps. This is crucial in understanding reaction mechanisms and pathways, such as those found in metabolic processes in biological systems or industrial chemical syntheses.
Practical Applications: Understanding Reactants and Products in Everyday Life
The concepts of reactants and products are not confined to the laboratory; they have widespread practical applications in our daily lives:
- Cooking: Cooking involves numerous chemical reactions, where food ingredients (reactants) are transformed into delicious meals (products) through processes like baking, frying, or boiling.
- Medicine: Drug development relies on understanding chemical reactions to design pharmaceuticals that interact with the body's systems. The drug itself is a reactant, and its interactions with biological molecules produce products that elicit therapeutic effects.
- Industrial Processes: Numerous industrial processes rely on controlled chemical reactions to produce valuable materials. From manufacturing plastics to refining petroleum, reactants are carefully chosen and reactions monitored to optimize product yield and quality.
- Environmental Chemistry: Understanding reactants and products is vital in environmental science, such as studying the effects of pollutants (reactants) on ecosystems and analyzing the formation of harmful byproducts (products) in pollution processes.
Common Misconceptions about Reactants and Products
Several common misconceptions can arise when dealing with reactants and products:
- Reactants always disappear completely: In some reactions, particularly equilibrium reactions, reactants do not completely disappear; instead, they reach a state of equilibrium where both reactants and products coexist.
- Products are always stable: While products are typically more stable than reactants, some products might be highly reactive and undergo further reactions.
- One reactant always yields one product: Many reactions yield multiple products, particularly complex organic reactions.
Conclusion
The distinction between reactants and products is fundamental to understanding chemical reactions and their applications across various fields. While seemingly a simple concept, the depth of knowledge related to reactants and products is vast and continues to be an area of extensive research and development. Mastering the understanding of reactants and products forms the groundwork for a deep comprehension of chemical principles and their impact on the world around us. By carefully examining the characteristics and behavior of reactants and products in various contexts, we can unlock the secrets of chemical transformations and utilize this knowledge for the betterment of science and technology.
Latest Posts
Latest Posts
-
Common Multiples Of 3 And 10
Mar 15, 2025
-
How Many Grams Are In 1 5 Pounds
Mar 15, 2025
-
How Many Cups Of Water Is 20 Oz
Mar 15, 2025
-
184 Out Of 200 As A Percentage
Mar 15, 2025
-
What Is 14 15 As A Percent
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between A Product And A Reactant . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
