What Is The Difference Between Electronegativity And Ionization Energy
Kalali
Mar 22, 2025 · 6 min read
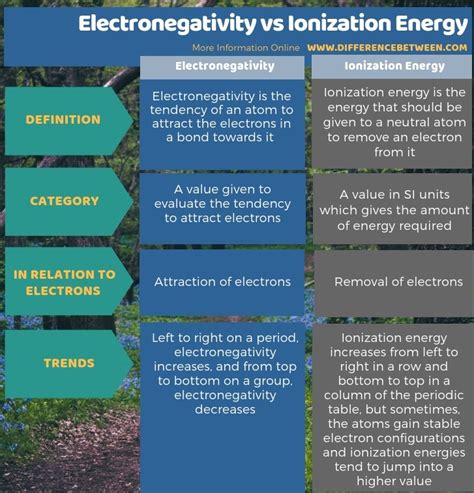
Table of Contents
What's the Difference Between Electronegativity and Ionization Energy?
Understanding the behavior of atoms and their interactions is fundamental to chemistry. Two key properties that govern this behavior are electronegativity and ionization energy. While both relate to an atom's ability to hold onto or attract electrons, they represent distinct concepts. This article will delve deep into the differences between electronegativity and ionization energy, exploring their definitions, trends within the periodic table, and practical applications.
Electronegativity: The Tug-of-War for Electrons
Electronegativity measures an atom's ability to attract electrons within a chemical bond. It's a relative property, meaning we compare the electronegativity of one atom to another within a molecule. Think of it as a tug-of-war: the atom with higher electronegativity pulls the shared electrons closer to itself. This doesn't necessarily mean it completely takes the electron; instead, it influences the electron density distribution within the bond.
Understanding the Scale
Electronegativity is typically represented on a scale, with the most electronegative element, fluorine (F), assigned a value of 4.0 (on the Pauling scale, the most commonly used). Other elements are ranked relative to fluorine. Elements with lower electronegativity values have a weaker pull on shared electrons.
Trends in the Periodic Table
Electronegativity generally increases across a period (from left to right) and decreases down a group (from top to bottom) in the periodic table.
-
Across a period: As you move across a period, the number of protons in the nucleus increases, resulting in a stronger positive charge attracting the electrons more strongly. The atomic radius generally decreases across a period, bringing the valence electrons closer to the nucleus. This combined effect leads to higher electronegativity.
-
Down a group: As you move down a group, the number of electron shells increases, shielding the valence electrons from the positive charge of the nucleus. The atomic radius increases significantly, placing the valence electrons further from the nucleus. This increased distance weakens the attractive force, resulting in lower electronegativity.
Consequences of Electronegativity Differences
The difference in electronegativity between atoms in a bond determines the bond's polarity:
-
Nonpolar covalent bonds: When the electronegativity difference is small (typically less than 0.5), the electrons are shared relatively equally between the atoms, resulting in a nonpolar covalent bond. Examples include bonds between two identical atoms (e.g., H-H, Cl-Cl).
-
Polar covalent bonds: When the electronegativity difference is moderate (between 0.5 and 1.7), the electrons are shared unequally, resulting in a polar covalent bond. The atom with higher electronegativity carries a partial negative charge (δ-), while the atom with lower electronegativity carries a partial positive charge (δ+). Examples include O-H bonds in water.
-
Ionic bonds: When the electronegativity difference is large (greater than 1.7), the atom with higher electronegativity essentially takes the electron from the other atom, forming ions and an ionic bond. Examples include NaCl (sodium chloride).
Ionization Energy: The Energy Cost of Electron Removal
Ionization energy (IE), also known as ionization potential, is the minimum energy required to remove an electron from a neutral gaseous atom in its ground state. This is a measure of how strongly an atom holds onto its electrons. The first ionization energy (IE₁) refers to the removal of the first electron, the second ionization energy (IE₂) refers to the removal of the second electron, and so on. Each successive ionization energy is higher than the previous one, as removing electrons leaves a more positively charged ion, making it harder to remove further electrons.
Trends in the Periodic Table
Similar to electronegativity, ionization energy follows specific trends in the periodic table:
-
Across a period: Ionization energy generally increases across a period. This is because the increasing nuclear charge attracts the electrons more strongly, making them harder to remove. The decreasing atomic radius also contributes to this trend.
-
Down a group: Ionization energy generally decreases down a group. The increased shielding effect of inner electrons and the larger atomic radius reduce the attraction between the nucleus and the valence electrons, making them easier to remove.
Factors Affecting Ionization Energy
Several factors influence an atom's ionization energy:
-
Nuclear charge: A higher nuclear charge leads to a stronger attraction for electrons, increasing ionization energy.
-
Atomic radius: A smaller atomic radius means electrons are closer to the nucleus, resulting in a stronger attraction and higher ionization energy.
-
Shielding effect: Inner electrons shield the outer electrons from the full positive charge of the nucleus. Increased shielding reduces the effective nuclear charge felt by the outer electrons, lowering ionization energy.
-
Electron configuration: Atoms with full or half-filled subshells have greater stability, and therefore higher ionization energies, than atoms with partially filled subshells.
Key Differences Summarized
| Feature | Electronegativity | Ionization Energy |
|---|---|---|
| Definition | Atom's ability to attract electrons in a bond | Minimum energy to remove an electron from a gaseous atom |
| Scale | Relative scale (Pauling scale, etc.) | Measured in kJ/mol or eV |
| Focus | Electron attraction within a molecule | Electron removal from an isolated atom |
| Bonding Type | Influences bond polarity (covalent, ionic, polar) | Related to the formation of positive ions (cations) |
| Periodic Trend | Increases across a period, decreases down a group | Increases across a period, decreases down a group |
Applications and Significance
Both electronegativity and ionization energy are crucial concepts in various areas of chemistry:
-
Predicting bond types: Electronegativity helps predict the type of bond formed between two atoms (ionic, covalent, polar covalent).
-
Understanding molecular polarity: The electronegativity difference between atoms dictates the polarity of a molecule, influencing its physical and chemical properties.
-
Explaining reactivity: Both ionization energy and electronegativity influence an atom's reactivity. Atoms with low ionization energies readily lose electrons, while those with high electronegativities readily gain electrons.
-
Spectroscopy: Ionization energy is directly measurable using techniques like photoelectron spectroscopy.
-
Material Science: Understanding these properties is crucial in designing new materials with desired electrical, optical, and mechanical properties. For example, the ionization energy of semiconductors dictates their electronic properties, affecting their use in electronics.
-
Biological Systems: The electronegativity of atoms like oxygen and nitrogen plays a critical role in the formation of hydrogen bonds, which are crucial for the structure and function of biological molecules like proteins and DNA.
Conclusion
While both electronegativity and ionization energy describe an atom's interaction with electrons, they represent different aspects. Electronegativity focuses on an atom's ability to attract electrons within a bond, influencing bond polarity. Ionization energy focuses on the energy required to remove an electron from an isolated atom, impacting its ability to form positive ions and influencing its reactivity. Understanding these distinct concepts is essential for comprehending chemical bonding, molecular properties, and the behavior of atoms and molecules in various contexts. Their trends within the periodic table offer a powerful tool for predicting and explaining chemical phenomena.
Latest Posts
Latest Posts
-
What Is 2 7 As A Percent
May 09, 2025
-
15 12 As A Mixed Number
May 09, 2025
-
Is Sand A Homogeneous Or Heterogeneous Mixture
May 09, 2025
-
Which Statement Describes Clean Air As A Natural Resource
May 09, 2025
-
What Percent Is 28 Out Of 35
May 09, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Electronegativity And Ionization Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.