What Is The Difference Between Ionization Energy And Electronegativity
Kalali
Mar 26, 2025 · 6 min read
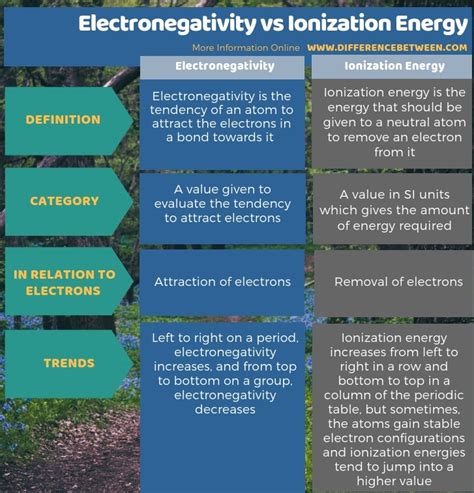
Table of Contents
What's the Difference Between Ionization Energy and Electronegativity?
Understanding the nuances of atomic behavior is crucial in chemistry. Two key properties that significantly influence how atoms interact are ionization energy and electronegativity. While both relate to an atom's ability to hold onto or attract electrons, they represent distinct concepts. This article delves deep into the differences between ionization energy and electronegativity, explaining their definitions, trends in the periodic table, and how they influence chemical bonding and reactivity.
Ionization Energy: The Energy to Remove an Electron
Ionization energy (IE) is the minimum amount of energy required to remove the most loosely bound electron from a neutral gaseous atom or ion. This process transforms a neutral atom into a positively charged ion (cation). It's important to note that this is always referring to a gaseous atom, as interactions with other atoms or molecules would significantly affect the energy required.
The ionization energy is not a single value for an atom; it's actually a series of values, representing the energy needed to remove successive electrons. The first ionization energy (IE₁) is the energy needed to remove the first electron, the second ionization energy (IE₂) is the energy required to remove the second electron from the singly charged cation, and so on. Each subsequent ionization energy is progressively higher than the previous one. This is because as electrons are removed, the remaining electrons experience a stronger effective nuclear charge (the net positive charge experienced by an electron), making it harder to remove subsequent electrons.
Trends in Ionization Energy Across the Periodic Table
Ionization energy generally follows predictable trends across the periodic table:
-
Increases across a period: As you move from left to right across a period (row) of the periodic table, the ionization energy increases. This is because the number of protons in the nucleus increases, leading to a stronger attraction for electrons and thus requiring more energy to remove one. The atomic radius generally decreases across a period, also contributing to the increased ionization energy.
-
Decreases down a group: As you move down a group (column) of the periodic table, the ionization energy decreases. This is due to the increasing atomic radius. The outermost electrons are further from the nucleus, experiencing a weaker effective nuclear charge, and therefore requiring less energy to remove. Shielding by inner electrons also plays a significant role here, reducing the attraction of the nucleus on valence electrons.
Factors Affecting Ionization Energy
Several factors influence the magnitude of ionization energy:
-
Nuclear charge: A higher nuclear charge leads to a stronger attraction for electrons, resulting in a higher ionization energy.
-
Atomic radius: A larger atomic radius means the outermost electrons are farther from the nucleus, experiencing weaker attraction and resulting in lower ionization energy.
-
Shielding effect: Inner electrons shield the outer electrons from the full positive charge of the nucleus, reducing the effective nuclear charge experienced by the outer electrons and lowering ionization energy.
-
Electron configuration: Atoms with stable electron configurations (e.g., noble gases) have significantly higher ionization energies than atoms with less stable configurations. Half-filled and fully-filled subshells also exhibit relatively high ionization energies due to extra stability.
Electronegativity: The Power to Attract Electrons
Electronegativity is a measure of the ability of an atom in a molecule to attract shared electrons to itself. Unlike ionization energy, which deals with a single isolated atom, electronegativity describes the behavior of an atom within a chemical bond. It reflects the relative pull an atom exerts on the electrons it shares with another atom. The higher the electronegativity, the greater the atom's ability to attract electrons within a bond.
Trends in Electronegativity Across the Periodic Table
Similar to ionization energy, electronegativity exhibits trends across the periodic table:
-
Increases across a period: Electronegativity generally increases as you move from left to right across a period. This is because of the increasing nuclear charge and decreasing atomic radius, similar to the trend observed in ionization energy.
-
Decreases down a group: Electronegativity generally decreases as you move down a group. This is due to the increasing atomic radius and the increased shielding effect, again mirroring the ionization energy trend.
The Pauling Scale: Measuring Electronegativity
Electronegativity values are usually expressed using the Pauling scale, a relative scale where fluorine (the most electronegative element) is assigned a value of 4.0. Other elements are assigned values relative to fluorine. While the exact values may vary slightly depending on the calculation method used, the relative trends remain consistent.
Factors Affecting Electronegativity
Several factors influence an atom's electronegativity:
-
Nuclear charge: A higher nuclear charge results in a stronger pull on shared electrons, leading to higher electronegativity.
-
Atomic radius: A smaller atomic radius leads to a stronger attraction for shared electrons, resulting in higher electronegativity.
-
Shielding effect: Inner electrons shield the outer electrons, reducing the effective nuclear charge and thereby lowering electronegativity.
Key Differences Between Ionization Energy and Electronegativity: A Summary
| Feature | Ionization Energy | Electronegativity |
|---|---|---|
| Definition | Energy required to remove an electron from an atom | Ability of an atom to attract shared electrons in a bond |
| Process | Removal of an electron | Attraction of shared electrons |
| System | Isolated gaseous atom | Atom within a chemical bond |
| Units | kJ/mol (kilojoules per mole) | Pauling scale (relative scale) |
| Trend across period | Increases | Increases |
| Trend down group | Decreases | Decreases |
The Relationship Between Ionization Energy and Electronegativity
While distinct, ionization energy and electronegativity are related. Elements with high ionization energies tend to have high electronegativities. This is because a strong hold on one's own electrons (high ionization energy) often correlates with a strong pull on shared electrons (high electronegativity). Both properties reflect the strength of the attraction between the nucleus and electrons. However, it's crucial to remember that they are not directly proportional; the relationship is more of a general trend, rather than a precise mathematical correlation.
Applications and Importance
Understanding ionization energy and electronegativity is crucial in various areas of chemistry:
-
Predicting Chemical Bonding: The difference in electronegativity between two atoms helps predict the type of bond they will form (ionic, covalent, polar covalent).
-
Determining Reactivity: Elements with low ionization energies tend to be more reactive as they readily lose electrons to form cations. Elements with high electronegativities tend to be highly reactive in gaining electrons to form anions.
-
Understanding Chemical Properties: These properties influence the physical and chemical properties of compounds, including melting points, boiling points, solubility, and reactivity.
-
Spectroscopy: Ionization energy is directly related to spectral lines observed in atomic emission spectroscopy.
-
Material Science: Understanding ionization energy and electronegativity are fundamental in designing and developing new materials with specific properties.
Conclusion
Ionization energy and electronegativity, although related, represent distinct atomic properties. Ionization energy focuses on the energy required to remove an electron from an isolated atom, while electronegativity describes an atom's ability to attract shared electrons within a bond. Both properties exhibit predictable trends across the periodic table and play vital roles in determining an element’s chemical behavior and the nature of its chemical bonds. A thorough understanding of these concepts is fundamental for mastering fundamental chemistry and its applications in diverse fields. By grasping these concepts, we gain a deeper appreciation for the intricate interplay of atomic forces that govern the world around us.
Latest Posts
Latest Posts
-
What Percent Of 40 Is 38
Mar 29, 2025
-
10 Is What Percentage Of 30
Mar 29, 2025
-
What Is 14 15 As A Percentage
Mar 29, 2025
-
Diagram Of Photosynthesis And Cellular Respiration
Mar 29, 2025
-
16 Inches Is How Many Feet
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Ionization Energy And Electronegativity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
