What Is The Difference Between Products And Reactants
Kalali
Mar 18, 2025 · 6 min read
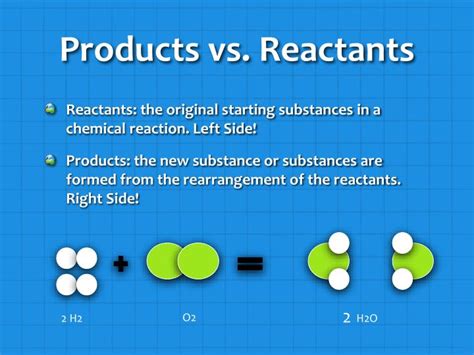
Table of Contents
What's the Difference Between Products and Reactants? A Deep Dive into Chemical Reactions
Understanding the difference between products and reactants is fundamental to grasping the core concepts of chemistry. While seemingly simple, a firm grasp of this distinction unlocks a deeper understanding of chemical reactions, stoichiometry, and the very nature of matter transformation. This comprehensive guide will delve into the definitions, characteristics, and practical applications of products and reactants, exploring the nuances that differentiate these key components of chemical processes.
Defining Reactants and Products: The Heart of Chemical Change
At its most basic, a chemical reaction involves the rearrangement of atoms to form new substances. The substances that undergo this transformation are called reactants, while the new substances formed are called products. This transformation is represented symbolically through chemical equations, where reactants are written on the left-hand side and products on the right-hand side, separated by an arrow indicating the direction of the reaction.
For example, in the combustion of methane (natural gas), the reaction can be written as:
CH₄ + 2O₂ → CO₂ + 2H₂O
Here, methane (CH₄) and oxygen (O₂) are the reactants, undergoing a chemical change. The carbon dioxide (CO₂) and water (H₂O) are the products, the new substances formed as a result of the reaction.
Key Characteristics of Reactants:
- Starting Materials: Reactants are the initial substances present before a chemical reaction begins. They possess specific chemical properties that dictate how they will interact and transform.
- Consumption During Reaction: During the reaction, reactants are consumed as their atoms are rearranged to form products. The amount of reactants dictates the maximum amount of product that can be formed (limiting reactant).
- Chemical Properties Change: Reactants undergo a significant change in their chemical properties during the reaction. Their chemical bonds are broken and reformed to create new substances with different properties.
Key Characteristics of Products:
- Resulting Substances: Products are the substances formed as a result of the chemical reaction. They have different chemical and often physical properties compared to the reactants.
- Formation During Reaction: Products are formed as a consequence of the rearrangement of atoms from the reactants. The amount of product formed depends on the amount of reactants and the efficiency of the reaction.
- New Chemical Properties: The products exhibit unique chemical properties, distinct from the reactants. These properties can be exploited for various applications, depending on the nature of the reaction and the products formed.
Visualizing the Transformation: Analogies and Examples
To further solidify the distinction between reactants and products, let's explore some analogies and real-world examples:
Analogy 1: Baking a Cake
Imagine baking a cake. The reactants would be the flour, sugar, eggs, butter, and other ingredients. Through the process of mixing and baking (the reaction), these reactants transform into the product: a delicious cake. The cake has entirely different properties than its individual ingredients.
Analogy 2: Rusting of Iron
Iron rusting is a classic example of a chemical reaction. Iron (Fe) and oxygen (O₂) from the air are the reactants. Over time, they react to form iron oxide (Fe₂O₃), or rust, which is the product. Rust is a different substance with different physical and chemical properties than the original iron.
Example 1: Photosynthesis
In photosynthesis, plants use carbon dioxide (CO₂) and water (H₂O) as reactants, along with sunlight as an energy source, to produce glucose (C₆H₁₂O₆) and oxygen (O₂) as products. This process is essential for life on Earth.
Example 2: Neutralization Reaction
When an acid reacts with a base, it's a neutralization reaction. For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), the reactants produce sodium chloride (NaCl), or table salt, and water (H₂O) as products. This reaction is an example of a simple acid-base neutralization.
Beyond the Basics: Reversible Reactions and Equilibrium
While the previous examples showcased simple reactions proceeding primarily in one direction, many chemical reactions are reversible. This means that the products can react to reform the reactants under specific conditions. These reactions are represented using a double arrow (⇌) in chemical equations.
For example, the conversion of nitrogen dioxide (NO₂) to dinitrogen tetroxide (N₂O₄) is a reversible reaction:
2NO₂ ⇌ N₂O₄
In reversible reactions, a state of equilibrium is often reached. At equilibrium, the rates of the forward reaction (reactants forming products) and the reverse reaction (products forming reactants) are equal. This means that the concentrations of reactants and products remain constant over time, even though the reactions are still occurring.
Understanding Stoichiometry: The Quantitative Relationship
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. By understanding stoichiometry, we can predict the amount of products that can be formed from a given amount of reactants, and vice versa. This is crucial in industrial processes, laboratory experiments, and various other applications.
Stoichiometric calculations rely on the balanced chemical equation, which shows the molar ratios between reactants and products. These ratios are essential for determining the limiting reactant, theoretical yield, and percent yield of a reaction.
Practical Applications: From Industry to Everyday Life
The understanding of reactants and products has far-reaching implications across various fields:
- Industrial Chemistry: Chemical industries rely heavily on understanding reactant ratios and product yields to optimize production processes and minimize waste. This is crucial in manufacturing fertilizers, plastics, pharmaceuticals, and countless other products.
- Environmental Science: Understanding chemical reactions is critical for assessing environmental impacts. For example, understanding the reactants and products involved in air pollution helps develop strategies for mitigating its effects.
- Medical Science: Biochemical reactions within our bodies involve intricate networks of reactants and products. Understanding these reactions is fundamental to developing new drugs and treatments for diseases.
- Food Science: The transformation of food ingredients during cooking involves numerous chemical reactions. Understanding the reactants and products helps create desirable textures, flavors, and nutritional properties in food.
Conclusion: A Foundation for Chemical Understanding
Differentiating between reactants and products is more than just memorizing definitions; it's about understanding the fundamental nature of chemical change. This distinction forms the cornerstone of numerous chemical concepts, from stoichiometry and equilibrium to the practical applications of chemistry across diverse fields. By mastering this fundamental concept, you open the door to a deeper appreciation of the complex and fascinating world of chemical reactions and their profound influence on our lives. The ability to accurately identify and predict the transformation of reactants into products is a skill that underpins much of chemical understanding and innovation. This knowledge allows us to engineer materials, create life-saving medicines, and develop sustainable technologies - a testament to the power of understanding the simple yet profound difference between reactants and products.
Latest Posts
Latest Posts
-
Is Sour Taste A Physical Property
Mar 19, 2025
-
How Many Kilos Are 20 Pounds
Mar 19, 2025
-
11 Out Of 30 As A Percentage
Mar 19, 2025
-
Cuanto Es 92 Grados Fahrenheit En Centigrados
Mar 19, 2025
-
How To Get Magnitude Of Force
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Products And Reactants . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
