What Is The Difference Between Reactants And Products
Kalali
Mar 12, 2025 · 6 min read
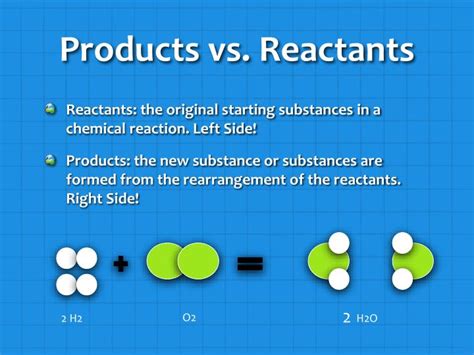
Table of Contents
What's the Difference Between Reactants and Products? A Deep Dive into Chemical Reactions
Understanding the difference between reactants and products is fundamental to grasping the core concepts of chemistry. This seemingly simple distinction underpins our comprehension of chemical reactions, the processes that shape our world from the rusting of iron to the complex metabolic pathways within our bodies. This article will delve deep into this crucial difference, exploring the definitions, roles, and representations of reactants and products, and examining their significance in various contexts.
Defining Reactants and Products: The Heart of Chemical Change
A chemical reaction is a process that leads to the transformation of one or more substances (the reactants) into one or more different substances (the products). This transformation involves the rearrangement of atoms, breaking and forming chemical bonds to create entirely new chemical entities with different properties. Think of it like baking a cake: your reactants are the flour, sugar, eggs, and other ingredients, while the cake itself is the product.
Reactants: These are the starting materials in a chemical reaction. They are the substances that undergo a chemical change, reacting with each other to form new substances. Reactants are typically written on the left side of a chemical equation, separated by plus signs. They are consumed during the reaction, their amounts decreasing as the reaction progresses.
Products: These are the substances formed as a result of a chemical reaction. They are the new substances created from the rearrangement of atoms within the reactants. Products are written on the right side of a chemical equation, also separated by plus signs. Their amounts increase as the reaction progresses.
Visualizing the Transformation: Chemical Equations
Chemical equations provide a concise and standardized way to represent chemical reactions. They use chemical formulas to symbolically represent the reactants and products, along with coefficients to indicate the relative amounts of each substance involved.
For example, consider the combustion of methane:
CH₄ + 2O₂ → CO₂ + 2H₂O
In this equation:
- CH₄ (Methane) and 2O₂ (Oxygen) are the reactants. They are the substances that react with each other.
- CO₂ (Carbon Dioxide) and 2H₂O (Water) are the products. They are the substances formed as a result of the reaction.
The arrow (→) indicates the direction of the reaction, showing the transformation from reactants to products. The coefficients (the numbers in front of the formulas) represent the stoichiometric ratios – the relative amounts of reactants and products involved in the balanced equation. This balanced equation ensures that the number of atoms of each element is the same on both sides, upholding the law of conservation of mass.
Beyond Simple Reactions: Exploring Complex Scenarios
While the methane combustion example illustrates a relatively straightforward reaction, many chemical processes are far more intricate. They may involve multiple steps, intermediate products, reversible reactions, and reaction mechanisms – complex sequences of elementary steps that constitute the overall reaction.
Reversible Reactions: Unlike the combustion of methane, some reactions are reversible, meaning that the products can react to reform the reactants. This is denoted by a double arrow (⇌) in the chemical equation. The extent to which a reaction proceeds in either direction is determined by the equilibrium constant, a value that reflects the relative amounts of reactants and products at equilibrium.
Reaction Mechanisms: These detail the step-by-step process of a reaction, often involving reactive intermediates that are neither reactants nor final products. Understanding reaction mechanisms provides crucial insights into reaction rates, activation energies, and the factors influencing reaction pathways.
Identifying Reactants and Products: Practical Applications
The ability to identify reactants and products is crucial in various fields:
- Industrial Chemistry: Optimizing industrial processes requires a precise understanding of reactants and products to control reaction yields, manage waste, and enhance efficiency.
- Environmental Science: Analyzing environmental pollutants involves identifying both the reactants (e.g., pollutants and atmospheric components) and products (e.g., reaction byproducts) to assess their impact and develop remediation strategies.
- Biochemistry and Medicine: Metabolic pathways, the complex sequences of chemical reactions that sustain life, rely on the precise interaction of reactants and products. Understanding these pathways is essential for developing new drugs and therapies.
- Analytical Chemistry: Quantitative analysis of chemical reactions requires accurate determination of reactant and product concentrations, enabling precise measurements and predictions.
Reactants and Products: The Key to Understanding Chemical Change
The distinction between reactants and products is paramount in chemistry. It forms the cornerstone of our understanding of chemical transformations and allows us to describe, predict, and manipulate chemical reactions across diverse fields. From the simple combustion of fuels to the intricate processes of life itself, recognizing the roles of reactants and products is essential for unlocking the mysteries of the chemical world and applying this knowledge to practical applications.
This fundamental understanding allows us to:
- Predict the outcome of chemical reactions: Knowing the reactants allows us to predict the likely products.
- Control reaction conditions: Manipulating the reactants and reaction conditions can be used to control the rate and yield of the reaction.
- Design new materials: By carefully selecting reactants, chemists can synthesize new materials with desirable properties.
- Understand biological processes: The intricate network of metabolic reactions within living organisms relies heavily on the interaction of reactants and products.
Exploring Reactant and Product Characteristics: A Deeper Dive
Reactants and products are not simply defined by their position in a chemical equation; they also possess distinct characteristics:
Reactant Characteristics:
- Initial state: Reactants exist in their initial state before the reaction begins. Their properties are well-defined before interaction.
- Consumption: Reactants are consumed during the reaction, their concentration decreasing over time.
- Reactivity: Reactants possess chemical properties that allow them to participate in the reaction. This reactivity is dictated by factors such as their electronic configuration and bond strengths.
- Limiting Reactants: In many reactions, one reactant is present in a smaller amount than needed to react completely with the other reactants. This is called the limiting reactant, and it determines the maximum amount of product that can be formed.
Product Characteristics:
- Formation: Products are formed during the reaction. Their properties are different from the reactants.
- Accumulation: Products accumulate as the reaction progresses, increasing in concentration over time.
- Properties: Products have distinct physical and chemical properties, different from those of the reactants. These differences may be in terms of color, state of matter, solubility, etc.
- Yield: The yield of a reaction refers to the amount of product formed relative to the amount of reactants used. Factors such as temperature, pressure, and catalyst presence significantly influence yield.
Beyond the Basics: Advanced Considerations
The concept of reactants and products extends beyond simple stoichiometric reactions. Advanced topics include:
- Catalysis: Catalysts accelerate reaction rates without being consumed themselves. They influence the reaction pathway, allowing for the formation of products with higher efficiency.
- Equilibrium: In reversible reactions, the concentrations of reactants and products reach a state of equilibrium where the forward and reverse reaction rates are equal.
- Thermodynamics: Thermodynamics governs the energy changes associated with chemical reactions, determining whether a reaction will proceed spontaneously or require energy input.
- Kinetics: Chemical kinetics studies the rates of chemical reactions, investigating factors like reactant concentration, temperature, and catalyst presence.
Understanding these advanced concepts provides a more complete and nuanced understanding of the dynamics of chemical reactions and the interplay between reactants and products.
Conclusion: Reactants and Products – The Building Blocks of Chemistry
The simple yet profound difference between reactants and products is the cornerstone of chemical understanding. This distinction underpins our ability to describe, predict, and manipulate chemical reactions, impacting various disciplines from industrial processes to biological systems. By mastering this fundamental concept and delving into the advanced considerations discussed, we gain a more comprehensive and powerful grasp of the fascinating world of chemistry. Continuous exploration of this topic is essential for anyone seeking to deepen their understanding of chemical transformations and their profound influence on our world.
Latest Posts
Latest Posts
-
How Many Days Is 72 Hours From Tuesday
Jun 30, 2025
-
How Many Cups Are In 3 Quarts Of Water
Jun 30, 2025
-
25 Cents A Minute For An Hour
Jun 30, 2025
-
In Music What Does Allegro Mean Math Answer Key Pdf
Jun 30, 2025
-
What Is 1 5 Of A Tablespoon
Jun 30, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Reactants And Products . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.