What Is The Empirical Formula For The Compound C6h12o6
Kalali
Mar 17, 2025 · 5 min read
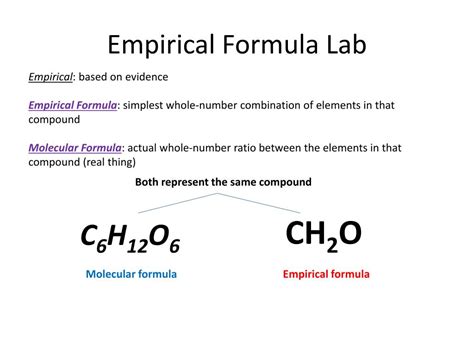
Table of Contents
What is the Empirical Formula for the Compound C₆H₁₂O₆? Understanding Molecular and Empirical Formulas
The question "What is the empirical formula for the compound C₆H₁₂O₆?" might seem deceptively simple at first glance. However, understanding the answer requires a solid grasp of the difference between molecular and empirical formulas, and the implications this has for representing chemical compounds. This article will delve deep into this topic, explaining the concepts clearly and providing a comprehensive understanding of empirical formulas and their relationship to molecular formulas, particularly using C₆H₁₂O₆ as our example.
Understanding Molecular and Empirical Formulas
Before we tackle the specific question, let's clarify the fundamental definitions:
Molecular Formula: This formula represents the actual number of atoms of each element present in a single molecule of a compound. It provides the exact composition of the molecule. For example, the molecular formula of glucose is C₆H₁₂O₆, indicating six carbon atoms, twelve hydrogen atoms, and six oxygen atoms in each glucose molecule.
Empirical Formula: This formula represents the simplest whole-number ratio of atoms of each element in a compound. It shows the ratio of elements, not the actual number of atoms in a molecule. It is the most reduced form of the molecular formula. To determine the empirical formula, you find the greatest common divisor (GCD) of the subscripts in the molecular formula and divide each subscript by that GCD.
Determining the Empirical Formula of C₆H₁₂O₆
Now, let's apply this to C₆H₁₂O₆, the molecular formula for glucose. To find the empirical formula, we need to determine the greatest common divisor of the subscripts 6, 12, and 6. The greatest common divisor of 6, 12, and 6 is 6.
Therefore, we divide each subscript by 6:
- C₆/₆ = C₁
- H₁₂/₆ = H₂
- O₆/₆ = O₁
This gives us the empirical formula: CH₂O.
This means that the simplest whole-number ratio of carbon, hydrogen, and oxygen atoms in glucose is 1:2:1. While the molecular formula accurately represents a single glucose molecule, the empirical formula represents the smallest unit that maintains the correct elemental ratio.
Why is the Empirical Formula Important?
The empirical formula is crucial in various aspects of chemistry:
-
Determining the Composition of Unknown Compounds: When analyzing an unknown compound, chemists often determine its empirical formula first through elemental analysis (techniques that measure the mass percentages of each element). This provides valuable information about the compound's composition.
-
Simplifying Calculations: Using the empirical formula can simplify calculations, particularly in stoichiometry (calculations involving chemical reactions). The molar mass (mass of one mole of a substance) is often calculated using the empirical formula, especially when the molecular formula is unknown.
-
Understanding Isomers: Many compounds share the same empirical formula but have different molecular formulas. These are called isomers. For example, several carbohydrates share the empirical formula CH₂O, but they differ in their molecular formulas (and thus their structures and properties). Glucose (C₆H₁₂O₆), fructose (C₆H₁₂O₆), and galactose (C₆H₁₂O₆) are all isomers; they have the same empirical formula but different arrangements of atoms, leading to different chemical behaviors.
-
Polymers: Polymers, long chains of repeating units (monomers), often have empirical formulas that represent the composition of the repeating unit. The actual molecular formula would be incredibly large and unwieldy, so the empirical formula is much more practical.
Distinguishing Between Empirical and Molecular Formulas: Practical Examples
Let's consider a few examples to highlight the difference between empirical and molecular formulas:
1. Acetic Acid:
- Molecular Formula: C₂H₄O₂
- Empirical Formula: CH₂O (Divide subscripts by 2)
2. Formaldehyde:
- Molecular Formula: CH₂O
- Empirical Formula: CH₂O (The molecular and empirical formulas are the same because the subscripts are already in the simplest whole-number ratio.)
3. Benzene:
- Molecular Formula: C₆H₆
- Empirical Formula: CH (Divide subscripts by 6)
These examples illustrate that the empirical formula represents the simplest ratio, while the molecular formula reflects the actual number of atoms in the molecule.
Further Applications and Significance
The concept of empirical formulas extends beyond simple organic compounds. In fields like materials science and inorganic chemistry, determining the empirical formula of a new material or compound is an essential first step in understanding its properties and potential applications. This can be crucial in the development of new catalysts, ceramics, and other advanced materials.
The precise determination of empirical formulas requires accurate analytical techniques, ensuring the accurate quantification of each element within the compound. Elemental analysis methods, often coupled with other spectroscopic techniques like mass spectrometry, are critical tools for this process. The data obtained from these methods informs the calculation of empirical formulas and aids in identifying the chemical composition of unknown samples.
Conclusion: The Importance of Context
While the empirical formula for C₆H₁₂O₆ is CH₂O, it's crucial to remember that this simplification loses important information about the actual molecular structure and properties. The empirical formula is useful for basic stoichiometric calculations and provides insight into the relative abundance of elements, but it does not represent the true molecular composition. For a complete and accurate understanding of glucose, the molecular formula C₆H₁₂O₆ is essential. The context in which the formula is used determines whether the empirical or molecular formula is more appropriate. Understanding the nuances of both formula types is essential for any student or professional working in the field of chemistry.
Latest Posts
Latest Posts
-
What Is 1 5 8 As A Decimal
Mar 17, 2025
-
3 Feet 6 Inches In Cm
Mar 17, 2025
-
What Is 26 Out Of 30 As A Percentage
Mar 17, 2025
-
How Many Feet Is 26 In
Mar 17, 2025
-
What Is 6 Out Of 20 As A Percentage
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Empirical Formula For The Compound C6h12o6 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
