What Is The Ideal Van't Hoff Factor For Glucose
Kalali
Mar 12, 2025 · 6 min read
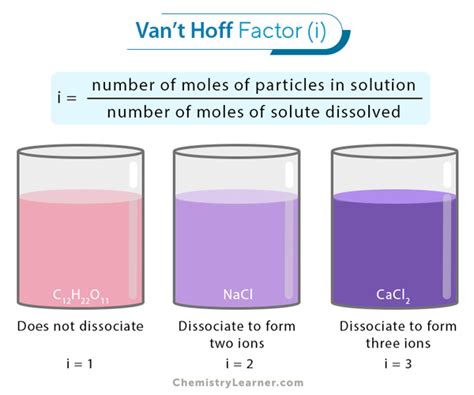
Table of Contents
What is the Ideal Van't Hoff Factor for Glucose? Understanding Colligative Properties
The van't Hoff factor (i) is a crucial concept in chemistry, particularly when dealing with colligative properties of solutions. These properties, such as boiling point elevation, freezing point depression, and osmotic pressure, depend solely on the concentration of solute particles, not their identity. The van't Hoff factor essentially corrects for the deviation from ideality caused by the dissociation or association of solute particles in solution. But what is the ideal van't Hoff factor for glucose, and why is understanding this important?
Understanding the Van't Hoff Factor
The van't Hoff factor represents the ratio of the actual number of particles in solution to the number of formula units initially dissolved. For a non-electrolyte that dissolves without dissociating or associating, the ideal van't Hoff factor is 1. This means one formula unit of solute dissolves to produce one particle in solution.
Examples of Ideal Van't Hoff Factors:
-
Glucose (C₆H₁₂O₆): Glucose is a non-electrolyte, meaning it does not dissociate into ions in solution. Therefore, its ideal van't Hoff factor is 1. One molecule of glucose dissolves to produce one particle in solution.
-
Sucrose (C₁₂H₂₂O₁₁): Similar to glucose, sucrose is a non-electrolyte with an ideal van't Hoff factor of 1.
-
Urea (CH₄N₂O): Urea is another example of a non-electrolyte with an ideal van't Hoff factor of 1.
Non-Ideal Behavior and Deviations from the Ideal Van't Hoff Factor
While the ideal van't Hoff factor provides a useful simplification, real-world solutions often deviate from ideal behavior. Several factors can influence the actual van't Hoff factor:
-
Ion Pairing: In solutions of strong electrolytes (like salts), ions can associate to form ion pairs, effectively reducing the number of independent particles in solution. This leads to a van't Hoff factor less than the theoretical value predicted by complete dissociation.
-
Association: Some molecules can associate in solution, forming larger aggregates. This decreases the number of particles and results in a van't Hoff factor less than 1.
-
Dissociation: Although glucose is a non-electrolyte and doesn't typically dissociate, under very specific and extreme conditions, it might undergo partial hydrolysis or other reactions, slightly altering the number of particles present. However, this is rare under normal physiological or laboratory conditions.
-
Concentration: At higher concentrations, intermolecular interactions between solute particles become more significant, leading to deviations from ideal behavior. The van't Hoff factor can be affected by concentration, often showing a decrease as concentration increases. This is especially noticeable in strong electrolyte solutions.
-
Solvent Interactions: The nature of the solvent can influence the extent of dissociation or association, and consequently, the van't Hoff factor.
Why is the Ideal Van't Hoff Factor for Glucose Important?
Understanding the ideal van't Hoff factor for glucose (and other non-electrolytes) is crucial for several reasons:
-
Accurate Calculation of Colligative Properties: The van't Hoff factor is directly incorporated into the equations used to calculate colligative properties. Using the correct value of i is essential for obtaining accurate results when determining boiling point elevation, freezing point depression, or osmotic pressure. For glucose, using i=1 ensures an accurate calculation.
-
Predicting Solution Behavior: Knowing that glucose has an ideal van't Hoff factor of 1 allows us to predict the behavior of glucose solutions with reasonable accuracy. We expect minimal deviations from ideality, unless extreme conditions are present.
-
Understanding Biological Systems: Glucose plays a vital role in biological systems. Understanding its behavior in solution, including its van't Hoff factor, is essential for comprehending processes such as osmosis in cells and the transport of glucose across cell membranes.
-
Pharmaceutical Applications: Many pharmaceutical formulations involve glucose or other non-electrolytes. Accurate calculations of colligative properties are crucial for ensuring the stability and efficacy of these products.
Determining the Van't Hoff Factor Experimentally
While the ideal van't Hoff factor for glucose is theoretically 1, it's possible to experimentally determine the actual van't Hoff factor for a specific glucose solution. This is typically done by measuring a colligative property, such as freezing point depression, and comparing the measured value to the value predicted using the ideal van't Hoff factor. Any deviation indicates a departure from ideality. However, for dilute glucose solutions, the experimental van't Hoff factor will be very close to 1, confirming its non-electrolyte nature.
Colligative Properties and the Van't Hoff Factor: Detailed Equations
The equations for calculating colligative properties incorporate the van't Hoff factor:
-
Freezing Point Depression: ΔTf = i * Kf * m, where ΔTf is the change in freezing point, Kf is the cryoscopic constant, and m is the molality of the solution.
-
Boiling Point Elevation: ΔTb = i * Kb * m, where ΔTb is the change in boiling point, Kb is the ebullioscopic constant, and m is the molality of the solution.
-
Osmotic Pressure: Π = i * MRT, where Π is the osmotic pressure, M is the molarity of the solution, R is the ideal gas constant, and T is the temperature in Kelvin.
These equations highlight the direct relationship between the van't Hoff factor and the magnitude of the colligative property. For glucose (i=1), the observed changes in freezing point, boiling point, and osmotic pressure are directly proportional to its concentration.
Advanced Considerations and Applications
The concept of the van't Hoff factor extends beyond simple solutions. It finds applications in more complex systems, such as:
-
Polymer Solutions: The van't Hoff factor can be adapted to describe the behavior of polymers in solution, where the effective number of particles may be influenced by the degree of polymerization and the interaction between polymer chains.
-
Ionic Liquids: In ionic liquids, ion pairing and other complex interactions can significantly impact the van't Hoff factor, leading to deviations from simple electrolyte behavior.
-
Colloidal Systems: Even in colloidal systems, where the solute particles are much larger than in typical solutions, the van't Hoff factor can be used to describe the influence of particle concentration on colligative properties.
Conclusion: The Significance of the Ideal Van't Hoff Factor for Glucose
The ideal van't Hoff factor for glucose is 1, reflecting its behavior as a non-electrolyte that dissolves without dissociating into ions. This understanding is fundamental to accurately calculating its colligative properties and predicting its behavior in solution. While deviations from ideality are possible under specific conditions, for most practical purposes, assuming a van't Hoff factor of 1 for glucose provides a reliable and accurate approach for understanding its role in various chemical and biological systems. Accurate calculation of colligative properties using the correct van't Hoff factor remains essential in numerous scientific and technological applications. The interplay between the van't Hoff factor and colligative properties provides a powerful tool for understanding solution behavior and its implications across diverse fields.
Latest Posts
Latest Posts
-
Is 27 A Prime Or Composite Number
Jul 10, 2025
-
What Is Double Of 1 4 Cup
Jul 10, 2025
-
Prepare Me A Body And I Will Redeem Man
Jul 10, 2025
-
How Many Inches Is A Meter Stick
Jul 10, 2025
-
Soundtrack To Step Up 2 The Streets
Jul 10, 2025
Related Post
Thank you for visiting our website which covers about What Is The Ideal Van't Hoff Factor For Glucose . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.