What Is The Molar Mass Of H2o
Kalali
Mar 18, 2025 · 5 min read
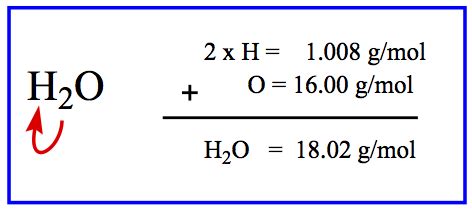
Table of Contents
What is the Molar Mass of H₂O? A Deep Dive into Water's Molecular Weight
Water, the elixir of life, is a simple yet profoundly important molecule. Understanding its properties, including its molar mass, is crucial across various scientific disciplines. This article delves deep into the calculation and significance of the molar mass of H₂O, exploring its implications in chemistry, biology, and beyond.
Understanding Molar Mass: The Foundation
Before we calculate the molar mass of water, let's establish a clear understanding of the concept itself. Molar mass, also known as molecular weight, represents the mass of one mole of a substance. A mole is a fundamental unit in chemistry, defined as the amount of a substance that contains Avogadro's number (approximately 6.022 x 10²³) of elementary entities (atoms, molecules, ions, etc.). Therefore, the molar mass essentially tells us the mass of 6.022 x 10²³ molecules of a given substance. It's typically expressed in grams per mole (g/mol).
The Importance of Molar Mass
Molar mass is a cornerstone of stoichiometry, allowing us to convert between mass, moles, and the number of particles. This is crucial for:
- Balancing chemical equations: Accurately determining the reactants and products involved in chemical reactions.
- Calculating reaction yields: Predicting the amount of product formed based on the amount of reactants.
- Determining solution concentrations: Calculating the molarity (moles per liter) of solutions.
- Understanding reaction rates: Relating the speed of a reaction to the concentration of reactants.
- Various applications in analytical chemistry: Quantifying substances through techniques like titration and spectrophotometry.
Calculating the Molar Mass of H₂O: A Step-by-Step Guide
Water (H₂O) is a simple molecule composed of two hydrogen atoms and one oxygen atom. To calculate its molar mass, we need the atomic masses of these elements. These values can be found on the periodic table:
- Hydrogen (H): Approximately 1.008 g/mol
- Oxygen (O): Approximately 16.00 g/mol
Now, let's perform the calculation:
- Molar mass of hydrogen (H): 1.008 g/mol * 2 (since there are two hydrogen atoms) = 2.016 g/mol
- Molar mass of oxygen (O): 16.00 g/mol * 1 (since there is one oxygen atom) = 16.00 g/mol
- Total molar mass of H₂O: 2.016 g/mol + 16.00 g/mol = 18.016 g/mol
Therefore, the molar mass of water (H₂O) is approximately 18.016 grams per mole.
Precision and Significant Figures
It's important to note that the atomic masses used in the calculation are approximate values. More precise values exist, depending on the isotopic composition of the elements. The number of significant figures used in the calculation should reflect the precision of the input values.
The Significance of Water's Molar Mass
The molar mass of water plays a vital role in many areas:
1. Chemistry and Biochemistry:
- Stoichiometric calculations: Determining the amounts of reactants and products in reactions involving water, such as hydration or dehydration reactions.
- Solution preparation: Accurately preparing solutions with specific molar concentrations.
- Understanding colligative properties: Properties of solutions that depend on the concentration of solute particles, such as freezing point depression and boiling point elevation, are directly related to the molar mass of the solute.
- Acid-base chemistry: Water's role as a solvent and its autoionization constant (Kw) are intimately linked to its molar mass and the concentration of H+ and OH- ions.
2. Environmental Science:
- Water quality analysis: Determining the concentrations of various substances dissolved in water samples often involves molar mass calculations.
- Hydrological studies: Understanding water flow and distribution in various environments relies on accurate calculations involving the mass and volume of water.
- Oceanography: Salinity and density calculations in marine environments are related to the molar mass of water and dissolved salts.
3. Biology and Physiology:
- Osmosis and diffusion: The movement of water across cell membranes is governed by osmotic pressure, which is related to the molar mass of water and dissolved substances.
- Metabolic processes: Numerous biochemical reactions involve water as a reactant or product; understanding the stoichiometry of these reactions requires knowledge of the molar mass.
- Plant physiology: Water transport in plants is influenced by factors related to water's molar mass and properties.
4. Industrial Applications:
- Chemical engineering: Designing and optimizing industrial processes involving water often necessitates precise calculations using molar mass.
- Pharmaceutical industry: Drug formulations and manufacturing often involve precise measurements of reactants, necessitating molar mass calculations.
- Food science: Water activity in food products is crucial for preservation and quality; it's related to the molar mass of water and other components.
Beyond the Basics: Isotopic Variations and Molar Mass
The molar mass of 18.016 g/mol is an average molar mass, considering the natural abundance of different isotopes of hydrogen and oxygen. Isotopes are atoms of the same element with varying numbers of neutrons. The most common isotopes are:
- ¹H (protium): The most abundant isotope of hydrogen.
- ²H (deuterium): A heavier isotope of hydrogen with one neutron.
- ¹⁶O: The most abundant isotope of oxygen.
- ¹⁷O and ¹⁸O: Less abundant isotopes of oxygen with additional neutrons.
The presence of these isotopes slightly alters the molar mass of water molecules containing them. For instance, water molecules containing deuterium (D₂O, heavy water) have a higher molar mass than those containing only protium. This difference has implications in various scientific and technological applications, such as nuclear magnetic resonance (NMR) spectroscopy and neutron scattering.
Conclusion: The Ubiquitous Importance of Water's Molar Mass
The seemingly simple calculation of the molar mass of water (H₂O) unveils a fundamental concept with wide-ranging implications across scientific disciplines. From basic stoichiometric calculations to complex biological processes and industrial applications, understanding the molar mass of water is essential for accurate measurements, predictions, and a deeper appreciation of this ubiquitous molecule's role in our world. Its precise value, accounting for isotopic variations, further refines our understanding of water's diverse behavior and applications in numerous fields. This knowledge is not merely an academic exercise; it's a crucial tool for advancing scientific understanding and technological innovation. The importance of grasping this seemingly straightforward concept underscores the profound interconnectedness of fundamental principles in chemistry and their impact on a broad spectrum of scientific and practical endeavors.
Latest Posts
Latest Posts
-
8 Ounces Is How Many Ml
Mar 18, 2025
-
10 Is What Percent Of 50
Mar 18, 2025
-
What Percent Of 60 Is 40
Mar 18, 2025
-
How Many Grams Is 200 Mg
Mar 18, 2025
-
What Is 16 Cm In Inches
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of H2o . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
