What Is The Oxidation State Of S In H2so4
Kalali
Mar 19, 2025 · 5 min read
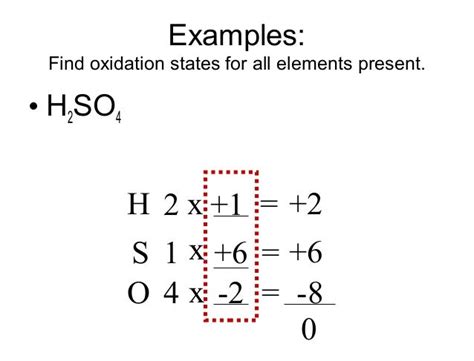
Table of Contents
What is the Oxidation State of S in H₂SO₄? A Deep Dive into Oxidation Numbers and Sulfur Chemistry
Determining the oxidation state of sulfur (S) in sulfuric acid (H₂SO₄) is a fundamental concept in chemistry, crucial for understanding its reactivity and properties. This article provides a comprehensive explanation, delving into the principles of oxidation states, the structure of H₂SO₄, and the calculations involved. We'll also explore related concepts and applications to solidify your understanding.
Understanding Oxidation States
Before we tackle the specific case of H₂SO₄, let's establish a clear understanding of oxidation states. The oxidation state, also known as oxidation number, is a hypothetical charge assigned to an atom in a molecule or ion, assuming that all bonds are completely ionic. It's a useful tool for tracking electron transfer in chemical reactions and predicting the reactivity of elements. Several rules govern the assignment of oxidation states:
- Rule 1: The oxidation state of an atom in its elemental form is always zero. For example, the oxidation state of S in S₈ (elemental sulfur) is 0.
- Rule 2: The oxidation state of a monatomic ion is equal to its charge. For example, the oxidation state of Na⁺ is +1, and the oxidation state of Cl⁻ is -1.
- Rule 3: The oxidation state of hydrogen (H) is usually +1, except when it's bonded to a less electronegative element, such as in metal hydrides (e.g., NaH), where it's -1.
- Rule 4: The oxidation state of oxygen (O) is usually -2, except in peroxides (e.g., H₂O₂) where it's -1, and in superoxides (e.g., KO₂) where it's -½.
- Rule 5: The sum of oxidation states of all atoms in a neutral molecule is zero.
- Rule 6: The sum of oxidation states of all atoms in a polyatomic ion is equal to the charge of the ion.
These rules are crucial for calculating oxidation states in complex molecules and ions. Let's apply them to determine the oxidation state of sulfur in sulfuric acid.
Calculating the Oxidation State of Sulfur in H₂SO₄
Sulfuric acid (H₂SO₄) is a strong mineral acid with a tetrahedral molecular geometry. To determine the oxidation state of sulfur, we'll use the established rules:
-
Hydrogen (H): Each hydrogen atom has an oxidation state of +1 (Rule 3). Since there are two hydrogen atoms, their total contribution is +2.
-
Oxygen (O): Each oxygen atom has an oxidation state of -2 (Rule 4). Since there are four oxygen atoms, their total contribution is -8.
-
Sulfur (S): Let's denote the oxidation state of sulfur as 'x'.
-
Neutral Molecule: The sum of oxidation states in a neutral molecule must be zero (Rule 5). Therefore, we can set up the following equation:
(+2) + x + (-8) = 0
-
Solving for x:
x = 0 - 2 + 8 x = +6
Therefore, the oxidation state of sulfur (S) in H₂SO₄ is +6. This high oxidation state reflects sulfur's ability to lose six electrons, showcasing its strong oxidizing properties.
The Significance of Sulfur's +6 Oxidation State in H₂SO₄
The +6 oxidation state of sulfur in H₂SO₄ is highly significant because it dictates several key properties of the molecule:
-
Strong Acidity: The high oxidation state of sulfur leads to a highly polarized S-O bond, making the molecule a strong acid. The O-H bonds are easily broken, releasing protons (H⁺) in aqueous solutions.
-
Oxidizing Properties: Although sulfuric acid is not a strong oxidizing agent in dilute solutions, concentrated sulfuric acid exhibits strong oxidizing properties due to the high oxidation state of sulfur. It can oxidize various substances, such as metals and non-metals.
-
Dehydrating Agent: Concentrated sulfuric acid acts as a powerful dehydrating agent. Its high affinity for water allows it to remove water molecules from other compounds, facilitating dehydration reactions.
-
Reactivity: The high oxidation state renders sulfuric acid highly reactive, participating in various reactions, including esterification, sulfonation, and metal dissolution.
Related Concepts and Applications
Understanding the oxidation state of sulfur in H₂SO₄ is fundamental to grasping several related chemical concepts and applications:
-
Redox Reactions: The oxidation state of sulfur changes during redox reactions involving sulfuric acid. For example, in the reaction between concentrated sulfuric acid and copper, sulfur is reduced from +6 to +4, while copper is oxidized from 0 to +2.
-
Sulfur Chemistry: The various oxidation states of sulfur (+6, +4, +2, 0, -2) lead to a wide range of sulfur compounds with diverse properties and applications.
-
Industrial Applications: Sulfuric acid is one of the most important industrial chemicals, used extensively in the production of fertilizers, detergents, plastics, and various other materials. Its reactivity and properties, directly linked to the +6 oxidation state of sulfur, underpin its diverse applications.
-
Analytical Chemistry: The oxidation state of sulfur can be determined using various analytical techniques, including titrations and spectroscopic methods. Understanding its oxidation state is vital for accurate quantitative analysis.
Further Exploration: Other Sulfur-Containing Compounds
Let's briefly explore the oxidation states of sulfur in other common compounds to further illustrate this concept:
-
Sulfur Dioxide (SO₂): In SO₂, the oxidation state of sulfur is +4. This is a lower oxidation state than in H₂SO₄, resulting in different chemical properties. SO₂ is a reducing agent, and it is less acidic than H₂SO₄.
-
Hydrogen Sulfide (H₂S): In H₂S, the oxidation state of sulfur is -2. This is a strongly reducing agent, with a pungent odor.
Conclusion: Mastering Oxidation States for Chemical Understanding
Determining the oxidation state of sulfur in H₂SO₄ is a vital exercise that underscores the importance of oxidation numbers in understanding chemical behavior. The +6 oxidation state is directly responsible for sulfuric acid's strong acidity, oxidizing properties, and its diverse industrial applications. By understanding the principles governing oxidation states and applying them systematically, we can gain crucial insights into the properties and reactivity of various compounds. This knowledge is essential for students and professionals alike in fields ranging from analytical chemistry to industrial processes. Further exploration of redox chemistry and sulfur's diverse compounds will enhance your overall grasp of chemical principles.
Latest Posts
Latest Posts
-
Common Multiples Of 7 And 4
Mar 19, 2025
-
Cross Section Of A Rectangular Prism
Mar 19, 2025
-
What Is 2 3 In A Fraction
Mar 19, 2025
-
How Many Ounces In 1 4 Liters
Mar 19, 2025
-
4 1 2 As A Decimal
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Oxidation State Of S In H2so4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
