What Is The Second Step Of Protein Synthesis
Kalali
Mar 21, 2025 · 6 min read
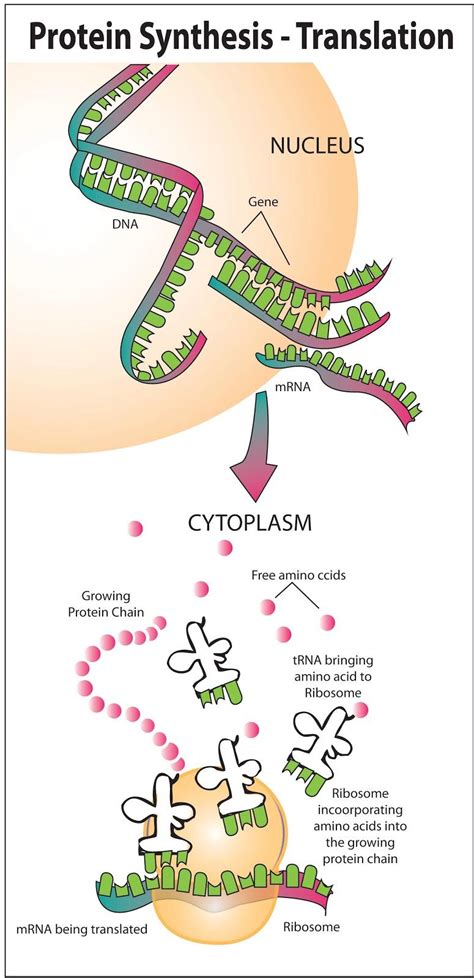
Table of Contents
What is the Second Step of Protein Synthesis? A Deep Dive into Translation
Protein synthesis, the fundamental process by which cells build proteins, is a two-step procedure: transcription and translation. While transcription creates an RNA copy of a gene's DNA sequence, translation takes that RNA message and uses it to assemble a chain of amino acids, ultimately forming a protein. This article delves deep into the second step, translation, exploring its intricacies, mechanisms, and significance in cellular function.
Understanding the Central Dogma: From DNA to Protein
Before we dive into the specifics of translation, let's briefly recap the central dogma of molecular biology: DNA → RNA → Protein. This illustrates the flow of genetic information within a cell. DNA, the master blueprint, holds the genetic code. Transcription copies a segment of this code into messenger RNA (mRNA). Finally, translation, the second step, deciphers the mRNA message and synthesizes the corresponding protein.
Translation: Decoding the mRNA Message
Translation occurs in the ribosomes, complex molecular machines found within the cytoplasm of cells (and also attached to the endoplasmic reticulum). The ribosome's primary role is to act as a protein synthesis factory, bringing together mRNA, transfer RNA (tRNA), and other crucial factors to build the polypeptide chain.
The Key Players in Translation
Several key players are involved in the intricate process of translation:
- Messenger RNA (mRNA): Carries the genetic code from DNA to the ribosome. The mRNA sequence is read in codons, three-nucleotide sequences that specify particular amino acids.
- Transfer RNA (tRNA): Acts as an adaptor molecule, carrying specific amino acids to the ribosome. Each tRNA molecule has an anticodon, a three-nucleotide sequence complementary to a specific mRNA codon.
- Ribosomes: Composed of ribosomal RNA (rRNA) and proteins, ribosomes provide the structural framework for translation and catalyze peptide bond formation. They have two subunits, a large and a small subunit, that come together during translation.
- Aminoacyl-tRNA synthetases: These enzymes attach the correct amino acid to its corresponding tRNA molecule, a crucial step ensuring accuracy in protein synthesis.
- Initiation, elongation, and termination factors: These proteins regulate the different stages of translation, ensuring the process proceeds smoothly and accurately.
The Three Stages of Translation
Translation is a highly regulated process divided into three main stages: initiation, elongation, and termination.
1. Initiation: This is the starting point of translation. It involves the assembly of the ribosome on the mRNA molecule, the recruitment of the initiator tRNA, and the positioning of the ribosome at the start codon (AUG).
* **Recognition of the mRNA:** The small ribosomal subunit binds to the mRNA molecule, scanning it until it encounters the start codon (AUG), which codes for methionine.
* **Initiator tRNA binding:** The initiator tRNA, carrying methionine, binds to the start codon within the P site (peptidyl site) of the small ribosomal subunit.
* **Large subunit joining:** The large ribosomal subunit joins the complex, forming the complete ribosome. This completes the initiation phase, ready for the next stage: elongation.
2. Elongation: This stage is where the polypeptide chain is actually built. It involves repeated cycles of codon recognition, peptide bond formation, and translocation.
* **Codon Recognition:** The next codon on the mRNA molecule moves into the A site (aminoacyl site) of the ribosome. A tRNA molecule carrying the amino acid specified by this codon then binds to the A site.
* **Peptide Bond Formation:** A peptide bond is formed between the amino acid in the A site and the growing polypeptide chain in the P site. This reaction is catalyzed by peptidyl transferase, an enzymatic activity of the large ribosomal subunit.
* **Translocation:** The ribosome moves one codon along the mRNA molecule. The tRNA in the P site (now carrying the shortened polypeptide chain) moves to the E site (exit site) and exits the ribosome, while the tRNA in the A site (now carrying the growing polypeptide chain) moves to the P site. This makes room for the next codon to enter the A site and repeat the cycle.
This cycle of codon recognition, peptide bond formation, and translocation continues until the ribosome encounters a stop codon.
3. Termination: This marks the end of translation. It involves the release of the completed polypeptide chain from the ribosome.
* **Stop Codon Recognition:** When a stop codon (UAA, UAG, or UGA) enters the A site, there is no tRNA that can recognize it. Instead, release factors bind to the A site.
* **Peptide Bond Hydrolysis:** Release factors stimulate the hydrolysis of the bond between the polypeptide chain and the tRNA in the P site, releasing the completed polypeptide.
* **Ribosome Dissociation:** The ribosome then dissociates into its subunits, ready to initiate translation of another mRNA molecule.
The Role of tRNA: The Adaptor Molecule
tRNA molecules are critical in translation, bridging the gap between the mRNA codons and the amino acids they specify. Each tRNA molecule has:
- Anticodon: A three-nucleotide sequence complementary to a specific mRNA codon.
- Amino acid attachment site: A site where the corresponding amino acid is attached.
The accuracy of amino acid attachment to the correct tRNA is crucial for the fidelity of protein synthesis. This is achieved by aminoacyl-tRNA synthetases, enzymes that specifically recognize both a tRNA molecule and its corresponding amino acid. They catalyze the formation of an ester bond between the amino acid and the tRNA.
Post-Translational Modifications
Once the polypeptide chain is released from the ribosome, it often undergoes several post-translational modifications. These modifications are crucial for the proper folding, function, and stability of the protein. Examples include:
- Protein folding: The polypeptide chain folds into a specific three-dimensional structure, determined by its amino acid sequence and interactions with chaperone proteins.
- Cleavage: Some proteins are synthesized as inactive precursors (proproteins or zymogens) that require cleavage to become active.
- Glycosylation: The addition of carbohydrate groups to the protein, influencing its function and stability.
- Phosphorylation: The addition of phosphate groups, commonly used as a regulatory mechanism.
Errors in Translation and their Consequences
While highly accurate, translation can sometimes lead to errors. These errors can have significant consequences, potentially leading to non-functional proteins or diseases. Errors can arise from:
- Mischarging of tRNAs: The incorrect attachment of an amino acid to a tRNA molecule.
- Frame-shift mutations: Insertions or deletions of nucleotides that alter the reading frame of the mRNA, leading to the synthesis of an entirely different protein.
- Nonsense mutations: Mutations that introduce premature stop codons, leading to the production of truncated proteins.
The Significance of Translation in Cellular Processes
Translation is a fundamental cellular process with far-reaching implications:
- Enzyme production: Enzymes, the catalysts of biochemical reactions, are proteins produced through translation.
- Structural protein synthesis: Structural proteins, such as collagen and keratin, provide structural support to cells and tissues.
- Signal transduction: Many proteins involved in signal transduction pathways are synthesized through translation.
- Gene regulation: Proteins involved in gene regulation are also produced through translation.
- Immune response: Antibodies, crucial components of the immune system, are proteins produced through translation.
Conclusion: The Central Role of Translation in Life
Translation, the second step of protein synthesis, is a remarkable and highly regulated process essential for life. The intricate interplay of mRNA, tRNA, ribosomes, and various other factors ensures the accurate synthesis of proteins, the workhorses of the cell. Understanding the intricacies of translation is crucial for comprehending cellular function, disease mechanisms, and the development of new therapeutic strategies. From the precise pairing of codons and anticodons to the post-translational modifications that fine-tune protein function, every aspect of translation highlights the elegance and complexity of biological systems. Further research into the mechanisms and regulation of translation promises to reveal even more profound insights into the workings of life itself.
Latest Posts
Latest Posts
-
How Many Feet In 50 Cm
Mar 27, 2025
-
What Is 40 F In Celsius
Mar 27, 2025
-
How Much Is 1 1 2 Cups In Oz
Mar 27, 2025
-
How Much Is 12 Oz Of Water
Mar 27, 2025
-
How Many Feet In 96 Inches
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about What Is The Second Step Of Protein Synthesis . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
