What Type Of Energy Is Created By Breaking The Bonds
Kalali
Mar 21, 2025 · 6 min read
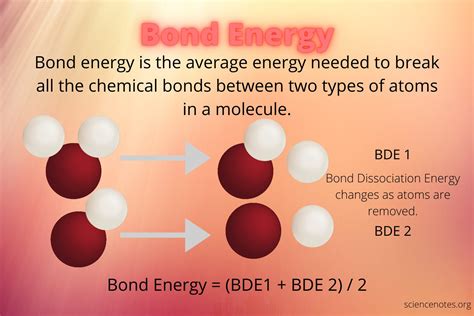
Table of Contents
- What Type Of Energy Is Created By Breaking The Bonds
- Table of Contents
- What Type of Energy is Created by Breaking Bonds?
- The Nature of Chemical Bonds and Their Energy
- Types of Chemical Bonds:
- Bond Energy:
- Energy Released Upon Bond Breaking: Different Forms
- 1. Heat Energy (Thermal Energy):
- 2. Light Energy (Radiant Energy):
- 3. Electrical Energy:
- 4. Mechanical Energy:
- 5. Sound Energy:
- Applications of Energy Released from Bond Breaking
- 1. Energy Production:
- 2. Industrial Processes:
- 3. Biological Systems:
- 4. Explosives:
- 5. Batteries and Fuel Cells:
- Factors Affecting Energy Released During Bond Breaking
- Conclusion: The Significance of Bond Breaking
- Latest Posts
- Latest Posts
- Related Post
What Type of Energy is Created by Breaking Bonds?
Breaking chemical bonds is a fundamental process in chemistry and physics, releasing energy in various forms. Understanding this energy release is crucial to comprehending numerous phenomena, from the combustion of fuels to the intricate processes within living organisms. This article delves deep into the nature of energy released during bond breakage, exploring its forms, applications, and implications.
The Nature of Chemical Bonds and Their Energy
Before discussing the energy released upon bond breakage, it's essential to understand the nature of chemical bonds themselves. Chemical bonds are the forces that hold atoms together to form molecules. These forces arise from the electrostatic interactions between the positively charged nuclei and the negatively charged electrons of the atoms involved. There are several types of chemical bonds, each with its own characteristics and bond energy:
Types of Chemical Bonds:
-
Covalent Bonds: These bonds form when atoms share electrons to achieve a more stable electron configuration. The strength of a covalent bond depends on the atoms involved and the number of electrons shared. Examples include the bonds in methane (CH₄) and water (H₂O). Breaking these bonds requires energy input, but breaking them releases energy as well.
-
Ionic Bonds: These bonds form through the electrostatic attraction between oppositely charged ions. One atom loses electrons (becoming a cation), while another gains electrons (becoming an anion). The strength of an ionic bond is determined by the charge and size of the ions. Examples include sodium chloride (NaCl) and magnesium oxide (MgO). Breaking ionic bonds typically requires significant energy.
-
Metallic Bonds: These bonds occur in metals, where valence electrons are delocalized and shared among a "sea" of electrons. This creates a strong bond that allows for the characteristic properties of metals, such as malleability and conductivity. Breaking metallic bonds requires considerable energy.
-
Hydrogen Bonds: These are relatively weak bonds that form between a hydrogen atom covalently bonded to a highly electronegative atom (like oxygen or nitrogen) and another electronegative atom. Hydrogen bonds play a crucial role in many biological systems, such as the structure of DNA and proteins. They are weaker than covalent, ionic, and metallic bonds.
Bond Energy:
Bond energy is defined as the amount of energy required to break one mole of a particular type of bond in the gaseous phase. It's typically expressed in kilojoules per mole (kJ/mol). Higher bond energy indicates a stronger bond, meaning more energy is required to break it. Conversely, breaking a stronger bond releases a larger amount of energy.
Energy Released Upon Bond Breaking: Different Forms
The energy released when bonds are broken doesn't appear in just one form. It manifests in various ways, depending on the specific reaction and the environment:
1. Heat Energy (Thermal Energy):
This is the most common form of energy released during bond breaking. When bonds break, the resulting atoms or molecules possess higher kinetic energy, leading to an increase in temperature. This is evident in combustion reactions, where the breaking of bonds in fuel molecules releases a significant amount of heat. Examples include burning wood, natural gas, or propane.
Example: The combustion of methane (CH₄) involves breaking the C-H bonds and the O=O bond in oxygen, releasing heat energy. This heat energy is then used to cook food, heat homes, or power engines.
2. Light Energy (Radiant Energy):
Certain chemical reactions, especially those involving excited atoms or molecules, release energy as light. This is called chemiluminescence. Fireflies, for instance, produce light through a chemiluminescent reaction. The energy released during bond breakage excites electrons to higher energy levels, and when these electrons return to their ground state, they emit photons of light.
Example: The reaction between luminol and hydrogen peroxide produces a characteristic blue glow as light energy is released.
3. Electrical Energy:
Bond breakage can also generate electrical energy. Batteries, for example, rely on chemical reactions involving bond breaking and formation to produce an electric current. The movement of ions during these redox reactions drives the flow of electrons, creating electrical energy.
Example: In a simple battery, zinc atoms lose electrons (oxidation) and copper ions gain electrons (reduction), creating an electrical potential difference.
4. Mechanical Energy:
In some instances, bond breakage can lead to the production of mechanical energy. Explosions, for example, involve the rapid release of energy due to the breaking of numerous bonds. This energy manifests as a sudden expansion of gases, producing mechanical work.
Example: The detonation of dynamite involves the rapid breaking of bonds in the explosive, releasing a significant amount of mechanical energy.
5. Sound Energy:
The rapid release of energy during certain bond-breaking processes can also generate sound energy. Explosions, as mentioned earlier, produce a loud sound due to the sudden expansion of gases and the vibration of surrounding matter. The breaking of bonds in a firework results in the characteristic bang.
Example: The breaking of chemical bonds in the propellant of a firework rocket generates sound energy.
Applications of Energy Released from Bond Breaking
The energy released from breaking chemical bonds is harnessed in numerous applications across various fields:
1. Energy Production:
Combustion of fossil fuels (coal, oil, natural gas) relies on the energy released from breaking carbon-hydrogen and carbon-carbon bonds. Nuclear fission involves breaking the bonds within atomic nuclei, releasing vast amounts of energy.
2. Industrial Processes:
Many industrial processes utilize the energy released during bond-breaking reactions. The production of cement, for example, involves high-temperature reactions where the breaking of bonds releases energy that drives the process.
3. Biological Systems:
Living organisms utilize the energy released during the breakdown of food molecules (such as glucose) to power various cellular processes. This energy, initially stored in chemical bonds, is converted into ATP (adenosine triphosphate), the primary energy currency of cells.
4. Explosives:
Explosives rely on the rapid breaking of bonds to release a tremendous amount of energy in a short period. This energy produces a shock wave and generates mechanical work, used for demolition, mining, and other applications.
5. Batteries and Fuel Cells:
Batteries and fuel cells convert the chemical energy released during bond breaking into electrical energy. They are used to power various electronic devices and vehicles.
Factors Affecting Energy Released During Bond Breaking
Several factors influence the amount of energy released during bond breaking:
-
Bond Strength: Stronger bonds release more energy upon breaking. The bond dissociation energy is a key factor in determining the overall energy released in a reaction.
-
Type of Reaction: Different reaction types (e.g., combustion, oxidation-reduction) release different amounts of energy.
-
Reaction Conditions: Temperature, pressure, and the presence of catalysts can significantly affect the rate and extent of a reaction, thereby influencing the energy released.
-
Reaction Mechanism: The specific pathway of a reaction (the mechanism) can also affect the energy released. Some mechanisms might involve intermediate steps that absorb or release additional energy.
Conclusion: The Significance of Bond Breaking
Breaking chemical bonds is a fundamental process that releases energy in various forms, driving numerous natural and technological processes. Understanding the type and quantity of energy released is essential for designing efficient energy production systems, developing new materials, and understanding biological processes. This knowledge is crucial across various disciplines, spanning from chemistry and physics to engineering and biology. The energy released through bond breaking is a fundamental force shaping our world, from the smallest cellular processes to the largest industrial applications. Further research into the intricacies of bond breaking will undoubtedly lead to advancements in many fields.
Latest Posts
Latest Posts
-
What Is 162 Cm In Ft
Mar 28, 2025
-
What Percent Of 29 Is 3
Mar 28, 2025
-
161 Cm In Inches And Feet
Mar 28, 2025
-
What Percentage Of 5 Is 4
Mar 28, 2025
-
How Many Oz Is 180 Ml
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Energy Is Created By Breaking The Bonds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
