Which Applies To The Collision Theory
Kalali
Mar 15, 2025 · 6 min read
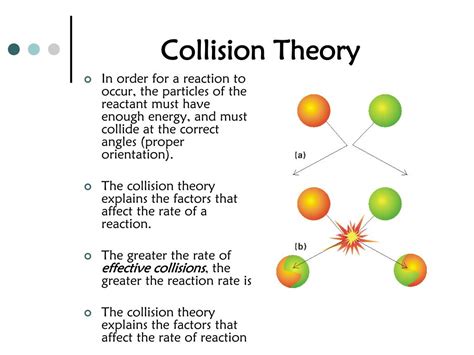
Table of Contents
Which Factors Apply to the Collision Theory? A Deep Dive into Reaction Rates
Collision theory is a fundamental concept in chemistry that explains how chemical reactions occur at the molecular level. It posits that reactions happen when reactant molecules collide with sufficient energy and the correct orientation. This seemingly simple statement underpins a wealth of complex phenomena affecting reaction rates. Understanding which factors apply to collision theory is crucial for predicting and manipulating reaction speeds in various applications, from industrial processes to biological systems. This article will explore the key factors influencing reaction rates as explained by collision theory.
The Core Principles of Collision Theory
Before delving into the influencing factors, let's solidify our understanding of collision theory's core principles:
-
Effective Collisions: A reaction only occurs if a collision between reactant molecules is effective. This means the collision must possess enough energy (activation energy) to break existing bonds and form new ones. Simply bumping into each other isn't enough; the molecules need to "collide" in a way that allows the rearrangement of atoms.
-
Activation Energy (Ea): This is the minimum energy required for an effective collision. It represents the energy barrier that reactant molecules must overcome to transform into products. Reactions with high activation energies are generally slower than reactions with low activation energies.
-
Orientation: Even with sufficient energy, a collision might not be effective if the molecules don't collide with the correct orientation. The atoms involved in bond breaking and forming must be positioned appropriately for the reaction to proceed. Think of it like trying to fit two puzzle pieces together – they need to be oriented correctly to connect.
Factors Affecting Reaction Rates According to Collision Theory
Numerous factors can influence the frequency and effectiveness of collisions, thus impacting reaction rates. These factors can be broadly categorized:
1. Concentration of Reactants
Higher concentration means more molecules are present in a given volume. This leads to a greater frequency of collisions, increasing the likelihood of effective collisions and hence accelerating the reaction rate. Imagine a crowded dance floor – more people (molecules) mean more collisions (interactions). Conversely, lower concentration reduces collision frequency, slowing down the reaction. This relationship is often expressed mathematically in rate laws.
2. Temperature
Temperature is directly related to the kinetic energy of molecules. Higher temperatures mean molecules move faster and possess greater kinetic energy. This increases both the frequency and the energy of collisions. A higher proportion of collisions will surpass the activation energy, leading to a significant increase in the reaction rate. The effect of temperature is often described by the Arrhenius equation, which quantifies the relationship between the rate constant and temperature.
3. Surface Area
This factor is primarily relevant for heterogeneous reactions (reactions involving reactants in different phases, like a solid and a liquid). Increasing the surface area of a solid reactant exposes more molecules to the other reactant, thus increasing the collision frequency and reaction rate. For example, a powdered solid reacts faster than a large lump of the same solid because the powder has a much larger surface area.
4. Pressure (for Gases)
For gaseous reactants, increasing the pressure increases the concentration of molecules in a given volume. This is analogous to the effect of increasing concentration; more molecules mean more frequent collisions, leading to a faster reaction rate. This is particularly important in industrial processes involving gaseous reactants.
5. Catalysts
Catalysts are substances that increase the rate of a reaction without being consumed in the process. They achieve this by providing an alternative reaction pathway with a lower activation energy. This means that more collisions will have sufficient energy to overcome the lower energy barrier, thus increasing the reaction rate. Catalysts do not affect the equilibrium position of a reversible reaction; they simply speed up the rate at which equilibrium is reached. Enzymes are biological catalysts crucial for life processes.
6. Nature of Reactants
The inherent properties of the reactants themselves play a role in determining the reaction rate. Some molecules react more readily than others due to factors such as:
-
Bond Strength: Stronger bonds require more energy to break, leading to a slower reaction rate.
-
Molecular Polarity: Polar molecules often react more readily than nonpolar molecules due to electrostatic interactions.
-
Steric Hindrance: The spatial arrangement of atoms within a molecule can hinder effective collisions if bulky groups obstruct the reactive sites. This reduces the likelihood of a favorable orientation for reaction.
Applying Collision Theory: Real-World Examples
Collision theory isn't just a theoretical concept; it's essential for understanding and manipulating reaction rates in various practical applications:
-
Industrial Chemistry: Optimizing reaction conditions (temperature, pressure, concentration) to maximize product yield and minimize reaction time is crucial in industrial chemical processes. Understanding collision theory allows chemical engineers to design efficient and cost-effective manufacturing processes.
-
Catalysis: The development of new and improved catalysts is a major area of research in chemistry. Catalysts are used extensively in various industries, from petroleum refining to the production of pharmaceuticals. Understanding how catalysts lower activation energy and increase reaction rates is central to this field.
-
Environmental Chemistry: Collision theory helps us understand the rates of atmospheric reactions, such as the formation of smog and acid rain. This knowledge is crucial for developing strategies to mitigate environmental pollution.
-
Biology: Enzymes, biological catalysts, are essential for all life processes. Their activity is governed by collision theory, explaining how they facilitate reactions within cells at rates compatible with life. Understanding enzyme kinetics, including factors affecting enzyme-substrate interactions, relies heavily on collision theory principles.
Beyond the Basics: Advanced Considerations
While the core principles of collision theory provide a solid foundation, several advanced concepts further refine our understanding:
-
Activated Complex (Transition State): The collision theory doesn't explicitly describe the intermediate state formed during a collision before the products are formed. The activated complex is a high-energy, unstable intermediate species that exists briefly before forming products or reverting to reactants.
-
Steric Factor (P): This factor accounts for the probability that colliding molecules have the correct orientation for a reaction to occur. It's often less than 1, indicating that only a fraction of collisions have the required orientation.
-
Collision Cross-Section: This represents the effective area of a molecule involved in a collision. Larger molecules generally have larger collision cross-sections, leading to more frequent collisions.
-
Quantum Mechanical Effects: At very low temperatures or with specific types of reactions, quantum mechanical tunneling can allow molecules to react even if they don't have sufficient energy to overcome the activation energy barrier.
Conclusion
Collision theory provides a powerful framework for understanding the factors that govern the rates of chemical reactions. By considering the concentration of reactants, temperature, surface area, pressure (for gases), the presence of catalysts, and the nature of reactants themselves, we can predict and manipulate reaction speeds in countless applications. While the basic principles are straightforward, a deeper understanding incorporates advanced concepts such as the activated complex, steric factor, and collision cross-section, which enhances the predictive power of the theory. Collision theory continues to be a cornerstone of chemical kinetics, providing valuable insights into the dynamic world of chemical transformations. Further research and exploration into these concepts will continue to refine our understanding of reaction mechanisms and lead to advancements across many scientific and technological fields.
Latest Posts
Latest Posts
-
What Is 26 Out Of 30 As A Percentage
Mar 17, 2025
-
How Many Feet Is 26 In
Mar 17, 2025
-
What Is 6 Out Of 20 As A Percentage
Mar 17, 2025
-
What Is Melting Point Of Glass
Mar 17, 2025
-
Cuanto Es El 30 Por Ciento De 500
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Which Applies To The Collision Theory . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
