Which Change Of Phase Is Exothermic
Kalali
Mar 12, 2025 · 6 min read
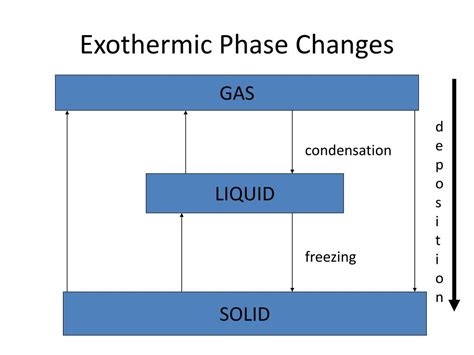
Table of Contents
Which Change of Phase is Exothermic? Understanding Exothermic Phase Transitions
Phase transitions are fundamental processes in nature, representing changes in the physical state of matter. These transitions involve the absorption or release of energy, a crucial aspect that dictates whether the process is endothermic (heat absorbing) or exothermic (heat releasing). While many associate melting and boiling with heat absorption, the reality is more nuanced. This article delves deep into the world of phase transitions, focusing specifically on which changes are exothermic, explaining the underlying principles and providing real-world examples.
Understanding Exothermic Processes
Before diving into specific phase transitions, it's vital to establish a clear understanding of exothermic processes. In simple terms, an exothermic process is any process that releases heat to its surroundings. This release of heat manifests as a decrease in the system's internal energy. The energy released is often in the form of heat, but it can also be in the form of light or sound. The key characteristic is that the system's energy content is lower after the process than before. This energy difference is often represented as a negative change in enthalpy (ΔH < 0).
Several familiar everyday examples illustrate exothermic processes:
- Combustion: Burning wood or fuel releases a significant amount of heat.
- Neutralization reactions: Mixing acids and bases generates heat as they react.
- Condensation: The transformation of water vapor into liquid water releases heat.
- Freezing: The conversion of a liquid into a solid releases heat.
Exothermic Phase Transitions: A Closer Look
Now, let's focus on phase transitions specifically. While many associate phase transitions with endothermic processes (like melting ice), several crucial changes are exothermic. The key is to understand the energy involved in the intermolecular forces within the substance.
The core principle is that any phase transition that involves a decrease in the freedom of movement of particles (molecules or atoms) is generally exothermic. This is because the particles are losing kinetic energy as they become more ordered. This lost kinetic energy is released as heat into the surroundings.
Let's examine the common exothermic phase transitions:
1. Deposition: From Gas to Solid
Deposition is the phase transition where a substance directly changes from a gaseous state to a solid state without passing through the intermediate liquid state. This transition is always exothermic. Imagine gas particles, moving freely and rapidly, suddenly losing their kinetic energy and becoming rigidly fixed in a solid structure (like snowflakes forming from water vapor). This significant decrease in kinetic energy translates to a release of heat.
Example: Frost forming on a cold surface is a classic example of deposition. The water vapor in the air directly transitions to ice crystals, releasing heat in the process.
2. Condensation: From Gas to Liquid
Condensation is a very familiar exothermic phase transition. Here, a substance transitions from a gaseous state to a liquid state. Gas molecules are highly energetic and move randomly at high speeds. During condensation, they lose kinetic energy, slow down, and become more closely packed together, forming a liquid. This decrease in kinetic energy manifests as a release of heat.
Example: The formation of dew on grass in the morning is a clear illustration of condensation. Water vapor in the air loses energy and condenses into liquid water droplets, releasing heat. Similarly, steam condensing on a cool surface is another excellent example. The heat released is what makes steam burns so severe.
3. Freezing: From Liquid to Solid
Freezing is the transition of a liquid substance into a solid state. In a liquid, the molecules are relatively free to move past each other, while in a solid, they are rigidly held in a fixed structure. As a liquid freezes, the molecules lose kinetic energy, becoming more ordered and less mobile. This loss of kinetic energy is released as heat, making freezing an exothermic process.
Example: Water freezing into ice is the quintessential example. The heat released during this process is why the surrounding environment may experience a slight temperature increase, although overall, the ice will be colder than the initial water.
Factors Affecting Exothermic Phase Transitions
Several factors influence the amount of heat released during exothermic phase transitions:
- Substance: Different substances have different intermolecular forces and thus release varying amounts of heat during phase transitions. For example, water releases a relatively large amount of heat during freezing compared to other substances.
- Mass: The amount of heat released is directly proportional to the mass of the substance undergoing the phase transition. A larger mass releases more heat.
- Temperature: The temperature difference between the substance and its surroundings influences the rate and extent of the exothermic phase transition. A larger temperature difference can lead to a faster and more significant heat release.
- Pressure: Pressure can also play a role, affecting the equilibrium point between different phases and ultimately influencing the heat released.
Distinguishing Between Endothermic and Exothermic Phase Transitions: A Summary Table
To avoid confusion, let's summarize the common phase transitions, categorizing them as either endothermic or exothermic:
| Phase Transition | Type | Heat Transfer | Description |
|---|---|---|---|
| Melting | Endothermic | Absorbs heat | Solid to liquid |
| Vaporization | Endothermic | Absorbs heat | Liquid to gas |
| Sublimation | Endothermic | Absorbs heat | Solid to gas |
| Freezing | Exothermic | Releases heat | Liquid to solid |
| Condensation | Exothermic | Releases heat | Gas to liquid |
| Deposition | Exothermic | Releases heat | Gas to solid |
Real-World Applications of Exothermic Phase Transitions
The principles of exothermic phase transitions have numerous practical applications:
- Cooling Systems: Condensation and freezing are utilized in refrigeration and air conditioning systems. The heat released during condensation is transferred to the surroundings, effectively cooling the enclosed space.
- Heating Systems: The heat released during condensation can be harnessed for heating purposes. For example, some heating systems utilize the heat generated from condensing steam.
- Material Science: Understanding exothermic phase transitions is critical in material science for controlling the properties of materials during processing. The controlled release of heat can be used to optimize the structure and properties of materials.
- Weather Phenomena: Deposition and condensation are responsible for many weather phenomena such as frost formation, dew formation, and cloud formation. The heat released during these processes plays a crucial role in atmospheric dynamics.
- Chemical Industry: Many industrial processes involve exothermic phase transitions, and understanding their thermodynamics is essential for efficient and safe operation.
Conclusion: Understanding the Importance of Exothermic Phase Transitions
Exothermic phase transitions, involving deposition, condensation, and freezing, are crucial processes in various natural phenomena and technological applications. Understanding these processes, including the factors influencing heat release, is fundamental across diverse scientific and engineering fields. This knowledge is key to designing efficient cooling systems, optimizing material properties, and interpreting weather patterns, among other applications. The release of heat during these transitions isn't just a byproduct; it's a vital aspect with far-reaching consequences and practical uses. By grasping the underlying principles, we can better understand and leverage the power of exothermic phase changes.
Latest Posts
Latest Posts
-
How Many Quarts In A 5 Gallon Bucket
Jul 05, 2025
-
How Far Is The Closest Ocean Beach To Me
Jul 05, 2025
-
How Long Is A China Man Joke
Jul 05, 2025
-
What Episode Does Ichigo Get His Powers Back
Jul 05, 2025
-
Pokemon Mystery Dungeon Red Rescue Team Codes
Jul 05, 2025
Related Post
Thank you for visiting our website which covers about Which Change Of Phase Is Exothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.