Which Characteristic Is Given By The Angular Momentum Quantum Number
Kalali
Mar 14, 2025 · 6 min read
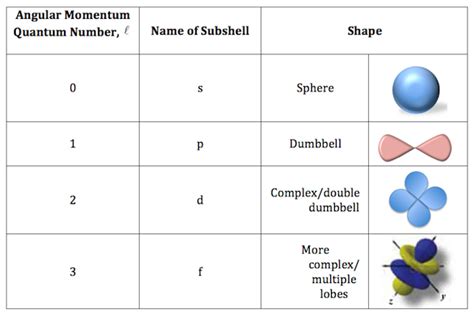
Table of Contents
Which Characteristic is Given by the Angular Momentum Quantum Number?
The angular momentum quantum number, denoted by l, is a crucial component in understanding the behavior of electrons within an atom. It dictates the shape of an electron's orbital and, consequently, its energy level and spatial distribution. While the principal quantum number (n) defines the overall energy level and size of the orbital, the angular momentum quantum number adds a layer of complexity, specifying the orbital's angular momentum and its three-dimensional form. This article delves deep into the characteristics conferred by l, exploring its significance in atomic structure, chemical bonding, and spectroscopy.
Understanding the Angular Momentum Quantum Number (l)
The angular momentum quantum number is an integer value that can range from 0 to n - 1, where n is the principal quantum number. This means that for a given energy level (n), there are n possible values of l. For instance:
- n = 1: l = 0 (only one possible value)
- n = 2: l = 0, 1 (two possible values)
- n = 3: l = 0, 1, 2 (three possible values) and so on.
Each value of l corresponds to a specific subshell within a given electron shell. These subshells are designated by letters, not numbers:
- l = 0: s subshell (spherical orbital)
- l = 1: p subshell (dumbbell-shaped orbitals)
- l = 2: d subshell (more complex, cloverleaf-shaped orbitals)
- l = 3: f subshell (even more complex shapes)
- l = 4: g subshell (and so on, although these higher subshells are less frequently encountered in common elements)
Orbital Shape and Spatial Distribution: The Defining Characteristic of l
The most significant characteristic determined by l is the shape of the atomic orbital. This shape directly influences the probability of finding an electron at a particular location around the nucleus.
s orbitals (l = 0)
s orbitals are spherically symmetrical. This means the probability of finding an electron is the same in all directions at a given distance from the nucleus. The electron cloud is concentrated closest to the nucleus. As n increases, the size of the s orbital increases, spreading the electron cloud further from the nucleus.
p orbitals (l = 1)
p orbitals have a dumbbell shape. There are three p orbitals within each p subshell, oriented along the x, y, and z axes (px, py, pz). Each p orbital has two lobes of electron density separated by a node (a region of zero electron probability) at the nucleus. Like s orbitals, p orbital size increases with increasing n.
d orbitals (l = 2)
d orbitals exhibit more complex shapes than s and p orbitals. There are five d orbitals in each d subshell, with various orientations and shapes including cloverleaf and donut-like configurations. These orbitals have two or more nodal planes.
f orbitals (l = 3) and beyond
f orbitals and higher orbitals possess even more intricate shapes with multiple nodes and lobes. Their complexity makes them more challenging to visualize, but their spatial distribution significantly influences the chemical properties of elements with electrons in these orbitals.
Energy Level and the Role of l
While the principal quantum number (n) primarily determines the overall energy level of an electron, the angular momentum quantum number (l) also plays a role, particularly in multi-electron atoms. In multi-electron atoms, electron-electron repulsions influence energy levels. For a given value of n, orbitals with lower l values generally have lower energy. This is known as the Aufbau principle, where electrons fill orbitals in order of increasing energy.
Therefore, within a given shell, the s orbital is the lowest in energy, followed by the p, then d, and so on. This energy ordering can slightly vary depending on the atom. This energy difference is crucial for understanding the behavior of electrons in chemical reactions and the formation of chemical bonds.
Magnetic Quantum Number (m<sub>l</sub>) and Orbital Orientation
The angular momentum quantum number (l) also determines the number of possible orientations of an orbital in space, which is described by the magnetic quantum number (m<sub>l</sub>). m<sub>l</sub> can have integer values ranging from -l to +l, including 0.
- For l = 0 (s orbital): m<sub>l</sub> = 0 (one orbital)
- For l = 1 (p orbital): m<sub>l</sub> = -1, 0, +1 (three orbitals)
- For l = 2 (d orbital): m<sub>l</sub> = -2, -1, 0, +1, +2 (five orbitals)
This means that the l value dictates the number of orbitals within a subshell, and thus the number of electrons that can occupy that subshell (each orbital can hold a maximum of two electrons according to the Pauli exclusion principle).
Implications in Chemical Bonding and Spectroscopy
The angular momentum quantum number's influence on orbital shape and energy has profound implications in chemical bonding and spectroscopy.
Chemical Bonding
The shape and spatial distribution of orbitals, governed by l, directly affect how atoms interact to form chemical bonds. For example:
- Overlap of orbitals: The formation of covalent bonds involves the overlap of atomic orbitals from different atoms. The degree of overlap depends on the shape and orientation of the orbitals. s orbitals, being spherically symmetric, readily overlap, while the directional nature of p, d, and f orbitals influences the geometry of the resulting molecule.
- Hybridization: The concept of hybridization, crucial in understanding molecular geometry, relies on the mixing of atomic orbitals with different l values. This allows for the formation of hybrid orbitals with specific shapes and directional properties.
Spectroscopy
Spectroscopic techniques, which analyze the interaction of electromagnetic radiation with matter, provide invaluable insights into the electronic structure of atoms. The absorption and emission of light by atoms involve transitions of electrons between different energy levels. These transitions are governed by the quantum numbers n and l. The energy differences between orbitals with different l values give rise to distinct spectral lines, enabling the identification of elements and the study of their electronic structure.
Advanced Considerations: Spin-Orbit Coupling and Relativistic Effects
At higher atomic numbers, relativistic effects become more pronounced. These effects can influence the energy levels of electrons and slightly alter the orbital shapes, particularly for orbitals with higher l values. Furthermore, spin-orbit coupling, the interaction between the electron's spin and its orbital angular momentum, can lead to further splitting of energy levels. These advanced phenomena demonstrate the intricate interplay between quantum numbers and atomic structure.
Conclusion: The Pivotal Role of l in Atomic Structure
The angular momentum quantum number (l) is far more than a simple integer; it is a fundamental quantum number that defines the crucial characteristics of atomic orbitals. Its influence extends beyond the basic shape and energy of atomic orbitals to significantly impact electron distribution, chemical bonding behavior, and spectroscopic properties. Understanding the characteristics given by l provides essential knowledge for comprehending the intricate world of atomic structure and the properties of matter. From the simple spherical symmetry of s orbitals to the complex shapes of f orbitals, the variations in orbital forms, dictated by l, form the very foundation of chemical behavior and physical properties observed across the periodic table. The multifaceted nature of l's influence serves as a reminder of the profound elegance and complexity inherent in the quantum world.
Latest Posts
Latest Posts
-
How Many Cups In 48 Fl Oz
Mar 14, 2025
-
55 Degrees Celsius Converted To Fahrenheit
Mar 14, 2025
-
A Corporation Must Appoint A President Chief Executive Officer
Mar 14, 2025
-
Is Roasting A Marshmallow A Chemical Change
Mar 14, 2025
-
Common Multiples Of 3 4 And 5
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Which Characteristic Is Given By The Angular Momentum Quantum Number . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
