Which Complex Carbohydrate Contains Only A 1 4 Glycosidic Linkages
Kalali
Mar 12, 2025 · 6 min read
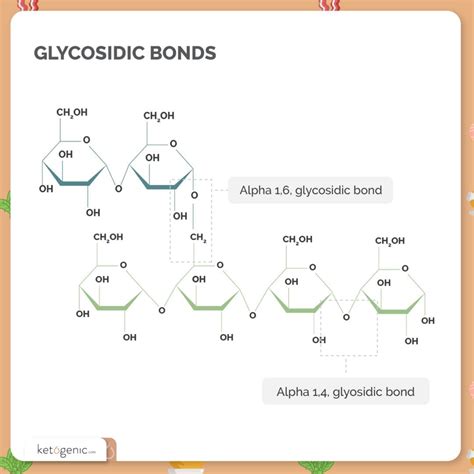
Table of Contents
Which Complex Carbohydrate Contains Only α-1,4 Glycosidic Linkages?
Understanding the intricacies of carbohydrate structures is crucial for appreciating their diverse roles in biological systems. While many complex carbohydrates boast a variety of glycosidic linkages, creating complex branched structures, some exhibit a simpler architecture. This article delves into the fascinating world of complex carbohydrates, focusing specifically on those featuring only α-1,4 glycosidic linkages. We'll explore their structure, function, and importance in various biological processes.
Deconstructing Carbohydrates: Glycosidic Linkages and Their Significance
Carbohydrates, the body's primary source of energy, are classified into monosaccharides (simple sugars), disaccharides (two monosaccharides joined), oligosaccharides (3-10 monosaccharides), and polysaccharides (long chains of monosaccharides). The crucial element linking these monosaccharide units is the glycosidic bond, a covalent bond formed between the hemiacetal or hemiketal group of a saccharide and the hydroxyl group of another molecule (often another saccharide). The glycosidic linkage is specified by the carbon atoms involved and the stereochemistry (α or β) of the anomeric carbon.
The α-1,4 glycosidic linkage signifies a bond between the carbon atom at position 1 (the anomeric carbon) of one monosaccharide and the carbon atom at position 4 of another monosaccharide, with the α-configuration at the anomeric carbon. This seemingly simple detail significantly impacts the resulting polysaccharide's structure and function.
Amylose: The Prime Example of α-1,4 Glycosidic Linkage
The most prominent example of a complex carbohydrate containing only α-1,4 glycosidic linkages is amylose. Amylose constitutes approximately 20-30% of starch, a major storage polysaccharide in plants. Starch, found abundantly in grains, tubers, and other plant parts, serves as a vital energy reserve for plants.
Amylose Structure: A Linear Chain of Glucose Units
Amylose is a linear polymer composed solely of α-D-glucose units linked together via α-1,4 glycosidic bonds. This linear arrangement leads to a helical structure, stabilized by hydrogen bonds within the chain. This helix is not rigidly fixed; it's dynamic and can adopt different conformations depending on the surrounding environment.
Amylose's Role in Energy Storage and Plant Physiology
The role of amylose in plants is primarily as an energy reserve. The α-1,4 linkages in amylose facilitate efficient energy storage. When plants require energy, enzymes break down the α-1,4 glycosidic bonds in amylose, releasing glucose molecules that can then be utilized for various metabolic processes. This process is crucial for plant growth, development, and survival.
Beyond energy storage, amylose also plays a role in plant cell structure and integrity. The physical properties of amylose, dictated by its helical structure and the α-1,4 linkages, contribute to the overall texture and consistency of plant tissues. This is particularly relevant in the context of food science and the textural properties of various plant-based foods.
Distinguishing Amylose from Other Starch Components: Amylopectin and Glycogen
It's crucial to differentiate amylose from other components of starch, particularly amylopectin. While amylose is a linear molecule with only α-1,4 linkages, amylopectin also contains α-1,6 glycosidic linkages, creating branch points along its structure. This branching introduces a more compact and readily accessible structure for enzymatic breakdown, making amylopectin more quickly digestible than amylose.
Another crucial distinction lies in comparing amylose with glycogen, the major storage polysaccharide in animals. Glycogen, like amylopectin, features both α-1,4 and α-1,6 glycosidic linkages, resulting in a highly branched structure. This extensive branching maximizes the number of non-reducing ends, making glycogen more readily available for energy mobilization compared to amylose.
The differences in structure between amylose, amylopectin, and glycogen directly impact their digestibility and utilization by living organisms. The linear structure of amylose with its sole reliance on α-1,4 linkages leads to a slower rate of digestion compared to the branched structures of amylopectin and glycogen. This difference has implications for blood glucose levels and overall metabolic health.
The Importance of α-1,4 Glycosidic Linkages in Digestion and Metabolism
The α-1,4 glycosidic bonds in amylose are targeted by specific enzymes during digestion. α-amylase, a key enzyme present in saliva and pancreatic juice, efficiently hydrolyzes these bonds, cleaving the amylose chain into smaller fragments, ultimately yielding glucose molecules for absorption and utilization by the body.
The efficiency of α-amylase in breaking down α-1,4 linkages ensures a reliable source of glucose for energy production. However, the relative resistance of amylose to digestion compared to amylopectin and glycogen influences the rate at which glucose enters the bloodstream. This slower release of glucose is advantageous in maintaining stable blood sugar levels, contributing to better overall metabolic control.
Beyond Energy Storage: Other Roles of α-1,4 Linked Polysaccharides
While amylose's primary function is energy storage, other polysaccharides with predominantly α-1,4 glycosidic linkages play diverse roles in various biological processes:
-
Cellulose: While cellulose primarily comprises β-1,4 glycosidic linkages, it’s crucial to understand its distinction from amylose. This difference in linkage configuration results in a significant structural contrast: cellulose forms rigid, linear fibers, contributing to plant cell wall strength, whereas amylose forms a flexible helix.
-
Dextrins: These are intermediate products of starch hydrolysis, often containing varying proportions of α-1,4 and α-1,6 linkages, but the α-1,4 bonds are predominant. Dextrins play roles in food processing and have applications in various industries.
-
Cyclodextrins: Formed via enzymatic degradation of starch, these cyclic oligosaccharides exhibit exclusively α-1,4 linkages. Their unique toroidal structures find applications in drug delivery, food science, and other areas.
The Future of Research: Exploring α-1,4 Linked Polysaccharides
Ongoing research continues to illuminate the diverse roles and potential applications of polysaccharides featuring α-1,4 glycosidic linkages. Areas of current investigation include:
-
Modified starches: Researchers are actively exploring modifications of starch to enhance its functionality in food products, improving texture, stability, and other desirable attributes. Understanding the intricacies of α-1,4 linkages is vital in these efforts.
-
Bio-based materials: The sustainable nature of polysaccharides makes them attractive candidates for creating bio-based materials, potentially replacing petroleum-derived polymers. Research focuses on harnessing the properties of α-1,4 linked polysaccharides to develop innovative bioplastics and other biomaterials.
-
Therapeutic applications: Investigations are underway to explore potential therapeutic applications of modified starches and other α-1,4 linked polysaccharides in drug delivery, wound healing, and other biomedical fields.
Conclusion: A Simple Linkage with Profound Implications
The α-1,4 glycosidic linkage, seemingly a simple structural detail, plays a crucial role in determining the properties and functions of a wide array of complex carbohydrates. Amylose, with its exclusive α-1,4 linkages, serves as a prime example of this pivotal role in energy storage and plant physiology. Further research will undoubtedly uncover even more diverse functionalities and applications of these remarkable molecules, highlighting the profound impact of this seemingly simple chemical bond. The understanding of α-1,4 linkages is not only crucial for understanding fundamental biological processes but also holds immense promise for developing innovative technologies across various sectors.
Latest Posts
Latest Posts
-
300 Inches Is How Many Feet
Mar 12, 2025
-
Is Formed When Ultraviolet Radiation Decomposes Chlorinated Hydrocarbon
Mar 12, 2025
-
5 Out Of 6 In Percentage
Mar 12, 2025
-
How Does An Igneous Rock Form Into A Sedimentary Rock
Mar 12, 2025
-
Cuanto Es 24 Onzas En Litros
Mar 12, 2025
Related Post
Thank you for visiting our website which covers about Which Complex Carbohydrate Contains Only A 1 4 Glycosidic Linkages . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
