Which Equation Represents An Oxidation Reduction Reaction
Kalali
Mar 17, 2025 · 6 min read
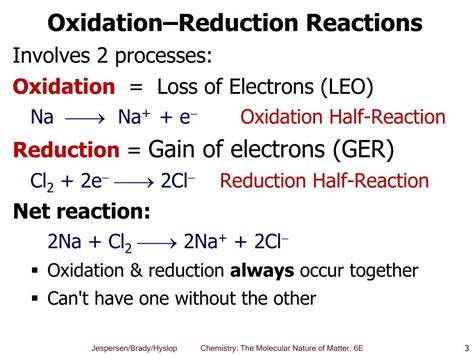
Table of Contents
Which Equation Represents an Oxidation-Reduction Reaction? A Comprehensive Guide
Oxidation-reduction reactions, also known as redox reactions, are fundamental chemical processes that involve the transfer of electrons between species. Understanding how to identify these reactions is crucial in various fields, from chemistry and biology to environmental science and materials science. This comprehensive guide will equip you with the knowledge and tools to confidently determine which equations represent redox reactions.
What are Oxidation-Reduction Reactions?
At the heart of a redox reaction lies the transfer of electrons. One species loses electrons (oxidation), while another species gains electrons (reduction). These two processes are always coupled; you can't have one without the other. Think of it like a seesaw – one side going up (oxidation) requires the other side going down (reduction).
Oxidation: The process of losing electrons. The oxidation state of the atom involved increases.
Reduction: The process of gaining electrons. The oxidation state of the atom involved decreases.
Oxidizing Agent: The species that accepts electrons, causing the reduction of another species. It gets reduced itself.
Reducing Agent: The species that donates electrons, causing the oxidation of another species. It gets oxidized itself.
Identifying Redox Reactions: Key Indicators
Several methods can help you identify whether an equation represents a redox reaction. Let's explore these techniques:
1. Changes in Oxidation States
The most reliable method involves tracking the oxidation states of atoms in the reactants and products. A change in oxidation state indicates a redox reaction.
-
Assigning Oxidation States: A set of rules helps assign oxidation states (also called oxidation numbers). These rules are based on electronegativity and the tendency of atoms to gain or lose electrons. Remember, the sum of oxidation states in a neutral molecule must equal zero, and in a polyatomic ion, it must equal the charge of the ion.
-
Identifying Changes: Compare the oxidation states of each element in the reactants and products. If any element's oxidation state changes, it's a redox reaction.
Example:
Consider the reaction: 2FeCl₂ + Cl₂ → 2FeCl₃
- Reactants: In FeCl₂, Fe has an oxidation state of +2, and Cl has -1. In Cl₂, Cl has an oxidation state of 0.
- Products: In FeCl₃, Fe has an oxidation state of +3, and Cl has -1.
Notice that the oxidation state of Fe increases from +2 to +3 (oxidation), while the oxidation state of Cl decreases from 0 to -1 (reduction). Therefore, this is a redox reaction.
2. Presence of Electron Transfer
Sometimes, the equation explicitly shows the transfer of electrons. This is a clear indicator of a redox reaction.
Example:
Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s)
This reaction can be written as two half-reactions:
- Oxidation: Zn(s) → Zn²⁺(aq) + 2e⁻
- Reduction: Cu²⁺(aq) + 2e⁻ → Cu(s)
The clear presence of electrons being transferred confirms this as a redox reaction. Zn is oxidized (loses electrons), and Cu²⁺ is reduced (gains electrons).
3. Changes in the Number of Bonds to Oxygen or Hydrogen
While not as definitive as oxidation state changes or explicit electron transfer, observing changes in the number of bonds to highly electronegative oxygen or electropositive hydrogen can often point towards a redox reaction.
-
Oxygen: An increase in the number of oxygen atoms bonded to an atom generally indicates oxidation. A decrease indicates reduction.
-
Hydrogen: An increase in the number of hydrogen atoms bonded to an atom generally indicates reduction. A decrease indicates oxidation.
Example:
The combustion of methane: CH₄ + 2O₂ → CO₂ + 2H₂O
Carbon in methane (CH₄) has a lower oxidation state than in carbon dioxide (CO₂), indicating oxidation of carbon. Simultaneously, oxygen in O₂ is reduced as it forms bonds with both carbon and hydrogen.
Common Types of Redox Reactions
Various types of redox reactions exist, each exhibiting unique characteristics:
1. Combustion Reactions
These reactions involve the rapid reaction of a substance with oxygen, producing heat and light. The fuel (usually containing carbon and hydrogen) is oxidized, and oxygen is reduced.
Example: The combustion of propane: C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
2. Corrosion Reactions
Corrosion is the gradual degradation of materials (usually metals) due to their reaction with the environment, primarily oxygen and water. The metal is oxidized, and oxygen is reduced.
Example: Rusting of iron: 4Fe + 3O₂ + 6H₂O → 4Fe(OH)₃
3. Single Displacement Reactions
In these reactions, a more reactive element displaces a less reactive element from a compound. The more reactive element is oxidized, and the less reactive element is reduced.
Example: Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
4. Disproportionation Reactions
These reactions involve a single element undergoing both oxidation and reduction simultaneously. The same element exists in different oxidation states in the products.
Example: 2Cu⁺(aq) → Cu²⁺(aq) + Cu(s)
5. Redox Titrations
Redox titrations are analytical techniques used to determine the concentration of an unknown solution using a redox reaction. The change in color of an indicator or electrochemical methods is employed to detect the equivalence point.
Distinguishing Redox Reactions from Non-Redox Reactions
Not all chemical reactions are redox reactions. Many reactions involve rearrangement of atoms without any electron transfer. Acid-base reactions and precipitation reactions are prime examples of non-redox reactions.
Acid-Base Reactions: These reactions involve the transfer of protons (H⁺) between an acid and a base. No change in oxidation states occurs.
Precipitation Reactions: These reactions involve the formation of a solid precipitate from the reaction of two aqueous solutions. No change in oxidation states occurs.
Advanced Concepts and Applications
Understanding redox reactions opens doors to advanced concepts and applications:
-
Electrochemistry: This field deals with the interconversion of chemical energy and electrical energy through redox reactions in electrochemical cells (batteries, fuel cells).
-
Biochemistry: Redox reactions play a vital role in biological processes, including respiration, photosynthesis, and metabolism. Electron transport chains are critical for energy generation in living organisms.
-
Environmental Chemistry: Redox reactions are crucial in understanding environmental processes such as water purification, pollution remediation, and atmospheric chemistry (e.g., ozone depletion).
-
Materials Science: Redox reactions are used in the synthesis and processing of many materials, including metals, ceramics, and polymers.
Conclusion
Identifying whether an equation represents a redox reaction requires careful attention to changes in oxidation states, electron transfer, and sometimes changes in the number of bonds to oxygen or hydrogen. Mastering these techniques allows one to understand a vast array of chemical and biological processes. The ability to analyze redox reactions is crucial for comprehending fundamental concepts across various scientific disciplines and for addressing important practical applications. This comprehensive guide serves as a solid foundation for further exploration into the fascinating world of oxidation-reduction reactions.
Latest Posts
Latest Posts
-
Are Vertical Asymptotes In The Numerator Or Denominator
Mar 17, 2025
-
What Fish Has Fins And Scales
Mar 17, 2025
-
What Is The Melting Point For Glass
Mar 17, 2025
-
What Is The Decimal Of 6 8
Mar 17, 2025
-
Cuanto Es El 30 Por Ciento De 1000
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Which Equation Represents An Oxidation Reduction Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
