Which Formula Represents An Unsaturated Hydrocarbon
Kalali
Mar 15, 2025 · 7 min read
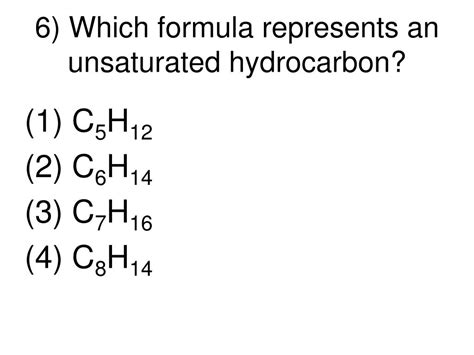
Table of Contents
Which Formula Represents an Unsaturated Hydrocarbon? Understanding Alkene and Alkyne Structures
Determining whether a chemical formula represents an unsaturated hydrocarbon involves understanding the fundamental principles of organic chemistry, specifically the concept of saturation and the structures of alkenes and alkynes. Hydrocarbons are organic compounds composed solely of carbon and hydrogen atoms. Unsaturated hydrocarbons contain at least one double or triple bond between carbon atoms, unlike saturated hydrocarbons (alkanes) which only have single bonds. This difference significantly impacts their chemical properties and reactivity.
Defining Saturation and Unsaturation in Hydrocarbons
The term "saturated" refers to a hydrocarbon molecule where every carbon atom is bonded to the maximum number of hydrogen atoms possible, utilizing only single bonds. This means each carbon atom forms four single bonds. The general formula for a saturated hydrocarbon (alkane) is C<sub>n</sub>H<sub>2n+2</sub>, where 'n' represents the number of carbon atoms. Examples include methane (CH<sub>4</sub>), ethane (C<sub>2</sub>H<sub>6</sub>), and propane (C<sub>3</sub>H<sub>8</sub>).
Conversely, "unsaturated" signifies that a hydrocarbon molecule contains at least one double bond (in alkenes) or a triple bond (in alkynes) between carbon atoms. This means some carbon atoms are not bonded to the maximum number of hydrogen atoms. The presence of these multiple bonds introduces regions of higher electron density, making these compounds more reactive than their saturated counterparts.
Alkenes: The Double Bond Story
Alkenes are unsaturated hydrocarbons characterized by the presence of at least one carbon-carbon double bond. The double bond consists of one sigma (σ) bond and one pi (π) bond. The pi bond, being less stable than the sigma bond, is responsible for the increased reactivity of alkenes. The general formula for an alkene is C<sub>n</sub>H<sub>2n</sub>, where 'n' is the number of carbon atoms. Notice that this formula has two fewer hydrogen atoms compared to the alkane formula (C<sub>n</sub>H<sub>2n+2</sub>) for the same number of carbon atoms, reflecting the presence of the double bond.
Identifying Alkenes in Chemical Formulas
To identify if a chemical formula represents an alkene, you need to check if the ratio of hydrogen to carbon atoms fits the C<sub>n</sub>H<sub>2n</sub> formula. For example:
- C<sub>2</sub>H<sub>4</sub> (Ethene): This fits the C<sub>n</sub>H<sub>2n</sub> formula (n=2), indicating an alkene.
- C<sub>3</sub>H<sub>6</sub> (Propene): This also follows the C<sub>n</sub>H<sub>2n</sub> formula (n=3), indicating an alkene.
- C<sub>4</sub>H<sub>8</sub> (Butene – several isomers exist): Again, this satisfies the C<sub>n</sub>H<sub>2n</sub> formula (n=4), indicating an alkene.
However, simply checking the formula isn't always sufficient. Cyclic hydrocarbons (hydrocarbons forming rings) can also have the same hydrogen-to-carbon ratio as alkenes. For instance, cyclopropane (C<sub>3</sub>H<sub>6</sub>) has the same formula as propene but is a cyclic alkane, not an alkene. Therefore, it's crucial to consider the structure beyond just the chemical formula.
Alkynes: The Triple Bond's Role
Alkynes are another class of unsaturated hydrocarbons distinguished by the presence of at least one carbon-carbon triple bond. This triple bond comprises one sigma (σ) bond and two pi (π) bonds. The presence of two pi bonds makes alkynes even more reactive than alkenes. The general formula for an alkyne is C<sub>n</sub>H<sub>2n-2</sub>, where 'n' is the number of carbon atoms. This formula shows four fewer hydrogen atoms compared to the alkane formula for the same number of carbon atoms, illustrating the impact of the triple bond.
Identifying Alkynes in Chemical Formulas
Similarly to alkenes, determining if a formula represents an alkyne involves examining the hydrogen-to-carbon ratio. Checking against the C<sub>n</sub>H<sub>2n-2</sub> formula is essential:
- C<sub>2</sub>H<sub>2</sub> (Ethyne): This fits the C<sub>n</sub>H<sub>2n-2</sub> formula (n=2), indicating an alkyne.
- C<sub>3</sub>H<sub>4</sub> (Propyne): This formula also adheres to C<sub>n</sub>H<sub>2n-2</sub> (n=3), suggesting an alkyne.
- C<sub>4</sub>H<sub>6</sub> (Butyne – several isomers exist): This follows the C<sub>n</sub>H<sub>2n-2</sub> formula (n=4), confirming an alkyne.
Again, structural considerations are vital. Cyclic hydrocarbons or those with multiple rings can sometimes possess the same hydrogen-to-carbon ratio as alkynes, emphasizing the need for a structural analysis to confirm the presence of a triple bond.
Beyond the Basic Formulas: Degree of Unsaturation
For more complex hydrocarbons with multiple double or triple bonds, or those containing rings, using the general formulas alone becomes insufficient. In these cases, the degree of unsaturation becomes a valuable tool. The degree of unsaturation represents the number of pi bonds and/or rings present in a molecule. It’s calculated using the following formula:
Degree of Unsaturation = [(2C + 2) + N - X - H] / 2
Where:
- C is the number of carbon atoms
- N is the number of nitrogen atoms
- X is the number of halogen atoms (F, Cl, Br, I)
- H is the number of hydrogen atoms
A degree of unsaturation of 1 indicates one double bond or one ring. A degree of unsaturation of 2 indicates two double bonds, one triple bond, or two rings, and so on.
Example Calculation of Degree of Unsaturation
Let's consider the molecule C<sub>5</sub>H<sub>8</sub>. Applying the formula:
Degree of Unsaturation = [(2 * 5 + 2) + 0 - 0 - 8] / 2 = 2
This indicates that C<sub>5</sub>H<sub>8</sub> has either two double bonds, one triple bond, or a combination of double bonds and rings. The actual structure needs further analysis to determine the specific arrangement.
Isomers and Structural Considerations
It's crucial to remember that the same chemical formula can represent different isomers (molecules with the same molecular formula but different arrangements of atoms). For example, C<sub>4</sub>H<sub>8</sub> can represent various isomers, including butene (with a double bond) and methylcyclopropane (a cyclic structure). Therefore, a mere chemical formula isn't enough to definitively confirm the presence of unsaturation; structural analysis is necessary for definitive identification.
Spectroscopy and Structural Elucidation
Modern techniques like nuclear magnetic resonance (NMR) spectroscopy and infrared (IR) spectroscopy play a critical role in determining the structure of organic molecules. These techniques provide detailed information about the bonding and arrangement of atoms, enabling scientists to distinguish between isomers and confirm the presence of double or triple bonds. NMR spectroscopy reveals details about the carbon and hydrogen environments, while IR spectroscopy identifies characteristic absorption bands associated with functional groups, such as C=C (alkene) and C≡C (alkyne) bonds.
Applications of Unsaturated Hydrocarbons
Unsaturated hydrocarbons, particularly alkenes and alkynes, are crucial in various applications due to their reactivity. They serve as building blocks in the synthesis of numerous organic compounds, including polymers, plastics, and pharmaceuticals. For instance:
- Ethene (C<sub>2</sub>H<sub>4</sub>): A crucial monomer in the production of polyethylene, a widely used plastic.
- Propylene (C<sub>3</sub>H<sub>6</sub>): Used in the synthesis of polypropylene, another important plastic.
- Ethyne (C<sub>2</sub>H<sub>2</sub>): Used in welding and cutting torches due to its high heat of combustion.
The reactivity of unsaturated hydrocarbons stems from the presence of the less stable pi bonds, enabling them to readily undergo addition reactions, where atoms or groups are added across the double or triple bond.
Conclusion: A Holistic Approach to Identifying Unsaturated Hydrocarbons
Identifying whether a chemical formula represents an unsaturated hydrocarbon necessitates a multi-faceted approach. While the general formulas C<sub>n</sub>H<sub>2n</sub> (alkenes) and C<sub>n</sub>H<sub>2n-2</sub> (alkynes) provide initial clues, they are not sufficient on their own. Considering the degree of unsaturation and structural analysis, potentially aided by spectroscopic techniques, is vital for definitive identification and differentiation between isomers. Understanding the concept of saturation, the unique properties of double and triple bonds, and the applications of unsaturated hydrocarbons are all crucial aspects of organic chemistry. The richness and complexity of hydrocarbon chemistry highlight the need for a holistic approach when analyzing their structures and properties.
Latest Posts
Latest Posts
-
What Is 1 5 8 As A Decimal
Mar 17, 2025
-
3 Feet 6 Inches In Cm
Mar 17, 2025
-
What Is 26 Out Of 30 As A Percentage
Mar 17, 2025
-
How Many Feet Is 26 In
Mar 17, 2025
-
What Is 6 Out Of 20 As A Percentage
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Which Formula Represents An Unsaturated Hydrocarbon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
