Which Has More Protons Sulfur Or Iodine
Kalali
Mar 13, 2025 · 5 min read
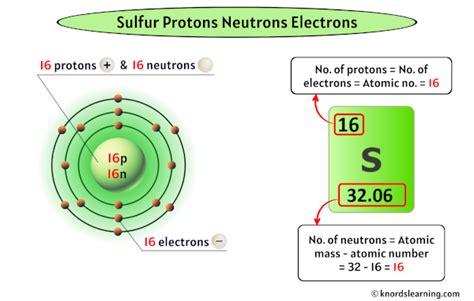
Table of Contents
Which Has More Protons: Sulfur or Iodine? A Deep Dive into Atomic Structure
Determining which element, sulfur or iodine, possesses more protons involves understanding fundamental concepts in chemistry, specifically atomic structure and the periodic table. This article will not only answer this question definitively but will also delve into the broader implications of proton number, exploring its relationship with other atomic properties and its significance in various scientific fields.
Understanding Atomic Structure: The Foundation of the Question
At the heart of every atom lies the nucleus, a dense central region containing two types of subatomic particles: protons and neutrons. Protons carry a positive charge, while neutrons are electrically neutral. Surrounding the nucleus is a cloud of negatively charged particles called electrons, which are significantly lighter than protons and neutrons.
The number of protons in an atom's nucleus defines its atomic number and uniquely identifies the element. This is a fundamental property that doesn't change under normal chemical conditions. The number of neutrons can vary, leading to different isotopes of the same element. Electrons, being relatively loosely bound, can be gained or lost in chemical reactions, resulting in the formation of ions.
Therefore, to determine whether sulfur or iodine has more protons, we simply need to consult the periodic table and find their respective atomic numbers.
The Periodic Table: A Roadmap to Atomic Properties
The periodic table is a beautifully organized arrangement of elements, ordered by increasing atomic number. Each element occupies a unique square containing its symbol, atomic number, and often, its atomic mass. This arrangement is not arbitrary; it reflects the periodic recurrence of similar chemical properties among elements. Elements with similar properties are grouped in columns called groups or families, while rows are known as periods.
Locating sulfur (S) and iodine (I) on the periodic table reveals their atomic numbers. Sulfur is located in period 3, group 16, and iodine is in period 5, group 17. Their atomic numbers are crucial for answering our central question.
Sulfur (S): Atomic Number 16
Sulfur's atomic number is 16. This signifies that every sulfur atom contains 16 protons in its nucleus. This number of protons dictates sulfur's chemical behavior and its position within the periodic table. Its 16 protons attract 16 electrons, resulting in a specific electron configuration that governs its reactivity and bonding capabilities.
Iodine (I): Atomic Number 53
Iodine's atomic number is 53. This indicates that every iodine atom possesses 53 protons in its nucleus. Like sulfur's proton number, this defines iodine's chemical characteristics, including its reactivity, bonding tendencies, and its position within the periodic table as a halogen. Its 53 protons attract 53 electrons, resulting in a different electron configuration compared to sulfur.
Comparing Proton Numbers: The Definitive Answer
Comparing the atomic numbers of sulfur (16) and iodine (53), we can definitively conclude that iodine (I) has significantly more protons than sulfur (S). The difference is substantial, highlighting the vast diversity in atomic structure across the periodic table. This difference in proton number leads to significant differences in their physical and chemical properties.
Implications of Proton Number: Beyond Simple Comparison
The difference in proton number between sulfur and iodine isn't merely an abstract numerical difference; it has profound implications for their properties and applications:
1. Chemical Reactivity:
The number of protons and consequently the electron configuration, largely determines an element's reactivity. Iodine, with its higher number of protons and outer electrons, exhibits different chemical behaviors compared to sulfur. Iodine is less reactive than sulfur, and its reactivity is influenced by its position as a halogen.
2. Physical Properties:
Proton number influences an element's physical properties such as melting point, boiling point, density, and electrical conductivity. Sulfur is a solid at room temperature, while iodine is a solid that readily sublimes (transitions directly from solid to gas). This difference is directly attributable to the differences in their atomic structures and the interatomic forces between their atoms.
3. Isotopes:
Both sulfur and iodine have several isotopes, which are variations of the same element differing only in the number of neutrons. The number of protons remains constant, defining the element. Isotopic variations can have significant implications in various applications, including medical imaging (e.g., radioactive iodine isotopes) and geological dating.
4. Applications:
The contrasting properties of sulfur and iodine lead to diverse applications. Sulfur is used extensively in the production of sulfuric acid, a crucial industrial chemical. Iodine, on the other hand, is used as an antiseptic, in the production of certain pharmaceuticals, and in various industrial processes.
Expanding the Understanding: Beyond Sulfur and Iodine
The principle of comparing atomic numbers to determine the number of protons applies to all elements in the periodic table. This fundamental concept underlies much of chemistry and other scientific disciplines, including nuclear physics and materials science. Understanding the relationship between proton number and elemental properties is crucial for various applications, including:
- Nuclear Chemistry: Understanding proton number is essential in nuclear reactions, including nuclear fission and fusion.
- Materials Science: The properties of materials are directly linked to the atomic structure and arrangement of their constituent elements, making proton number a critical parameter in material design and engineering.
- Analytical Chemistry: Various analytical techniques rely on the ability to identify elements based on their atomic number and other atomic properties.
The simple question of which element, sulfur or iodine, possesses more protons opens a door to a deeper understanding of atomic structure, chemical properties, and the periodic table, revealing the intricate relationships between seemingly simple numbers and the complex world of matter. The difference, a significant 37 protons, highlights the vast range of properties observed across the periodic table and the crucial role of proton number in determining an element's unique characteristics.
Latest Posts
Latest Posts
-
How Many Square Yards In One Acre
Jul 02, 2025
-
What Is 2 3 Of 3 Quarts
Jul 02, 2025
-
Courtesy Is Cumbersome To Them That Know It Not
Jul 02, 2025
-
How Far Is 220 Yards On A Track
Jul 02, 2025
-
What Is The Shortest Chapter In The Bible
Jul 02, 2025
Related Post
Thank you for visiting our website which covers about Which Has More Protons Sulfur Or Iodine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.