Which Is A General Representation Of A Secondary Amine
Kalali
Mar 29, 2025 · 6 min read
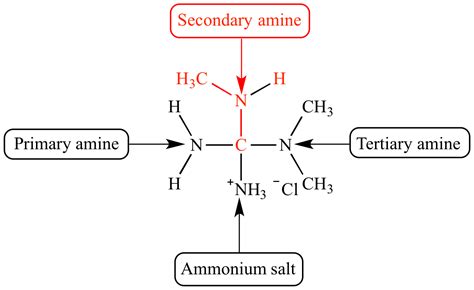
Table of Contents
Which is a General Representation of a Secondary Amine? A Deep Dive into Structure, Properties, and Reactions
Secondary amines represent a significant class of organic compounds with diverse applications in various fields, from pharmaceuticals to materials science. Understanding their general representation, properties, and reactivity is crucial for anyone working with or studying organic chemistry. This comprehensive article delves into the intricacies of secondary amines, providing a detailed overview of their structure, nomenclature, physical and chemical properties, and key reactions.
Understanding the Definition: What Makes a Secondary Amine?
Amines are organic derivatives of ammonia (NH₃), where one or more hydrogen atoms are replaced by alkyl or aryl groups. The classification of amines is based on the number of alkyl or aryl groups attached to the nitrogen atom:
- Primary (1°) amines: One alkyl or aryl group attached to the nitrogen. General formula: RNH₂.
- Secondary (2°) amines: Two alkyl or aryl groups attached to the nitrogen. General formula: R₂NH.
- Tertiary (3°) amines: Three alkyl or aryl groups attached to the nitrogen. General formula: R₃N.
This article focuses specifically on secondary amines, whose general representation is R₂NH, where 'R' represents an alkyl or aryl group (or a combination of both). The crucial feature differentiating a secondary amine is the presence of two carbon-containing substituents bonded to the nitrogen atom.
General Representation and Nomenclature of Secondary Amines
The general formula R₂NH provides a concise representation, but it's important to understand the diversity within this class. The 'R' groups can be identical (symmetrical secondary amines) or different (unsymmetrical secondary amines).
Examples of Secondary Amines:
- Dimethylamine (CH₃)₂NH: A symmetrical secondary amine with two methyl groups.
- Diethylamine (CH₃CH₂)₂NH: A symmetrical secondary amine with two ethyl groups.
- Methylethylamine CH₃NHCH₂CH₃: An unsymmetrical secondary amine with a methyl and an ethyl group.
- Diphenylamine (C₆H₅)₂NH: A symmetrical secondary amine with two phenyl groups.
- N-methylcyclohexylamine: An unsymmetrical secondary amine with a methyl group and a cyclohexyl group.
Nomenclature:
The naming of secondary amines follows IUPAC nomenclature rules. For symmetrical secondary amines, the alkyl or aryl group is named followed by "-amine" (e.g., dimethylamine). For unsymmetrical secondary amines, the smaller alkyl or aryl group is named as an N-substituent, preceded by "N-", followed by the name of the parent amine (e.g., N-methylcyclohexylamine). More complex structures might require more elaborate naming conventions involving locants and parent chain selection.
Physical Properties of Secondary Amines
The physical properties of secondary amines are significantly influenced by the nature of the alkyl or aryl groups attached to the nitrogen atom. Generally:
- Boiling Points: Secondary amines have higher boiling points compared to primary amines of similar molecular weight. This is due to stronger hydrogen bonding between the nitrogen lone pair of electrons and the hydrogen atoms of other amine molecules. However, tertiary amines have lower boiling points as they lack the N-H bond crucial for hydrogen bonding.
- Solubility: Lower molecular weight secondary amines are soluble in water due to hydrogen bonding. However, as the size of the alkyl or aryl groups increases, solubility decreases.
- Odor: Many secondary amines have characteristic fishy or ammoniacal odors.
- Basicity: Secondary amines are generally weaker bases than primary amines but stronger bases than tertiary amines. This difference in basicity is related to the electron-donating and steric effects of the alkyl or aryl groups. The presence of electron-withdrawing groups reduces basicity.
Chemical Properties and Reactions of Secondary Amines
Secondary amines undergo a variety of characteristic reactions, many of which are exploited in organic synthesis.
1. Basicity: The nitrogen atom in a secondary amine possesses a lone pair of electrons that can accept a proton (H⁺), acting as a Brønsted-Lowry base. This property allows them to react with acids to form salts. The basicity is influenced by the electronic and steric effects of the substituent groups.
2. Alkylation: Secondary amines can undergo alkylation reactions, adding another alkyl group to the nitrogen. However, the resulting product is a tertiary amine. This reaction requires strong alkylating agents.
3. Acylation: Reaction with acyl chlorides or acid anhydrides leads to the formation of amides. This reaction is a crucial step in the synthesis of many pharmaceuticals and polymers.
4. Diazotization: Secondary amines do not undergo diazotization in the same way as primary amines. Instead, they form N-nitroso compounds. This reaction is used in the synthesis of certain dyes and other specialized compounds.
5. Reaction with Carbonyl Compounds: Secondary amines react with aldehydes and ketones to form enamines. This reaction is widely used in organic synthesis for the preparation of heterocyclic compounds and other functionalized molecules.
6. Hofmann Elimination: When a quaternary ammonium salt (derived from a tertiary amine) undergoes Hofmann elimination, it generates an alkene. Although starting with a secondary amine, the process involves alkylation and then elimination.
Applications of Secondary Amines
The versatility of secondary amines makes them essential components in numerous industrial and biological processes:
- Pharmaceuticals: Many pharmaceuticals contain secondary amine functionalities, contributing to their biological activity. Examples include certain painkillers, antidepressants, and antihistamines.
- Polymers: Secondary amines are used as catalysts in the synthesis of polymers and as components in polymer structures, conferring specific properties like flexibility or reactivity.
- Dyes and Pigments: Certain secondary amines are used as intermediates in the synthesis of dyes and pigments, contributing to their color and stability.
- Agrochemicals: Secondary amines are found in some herbicides and pesticides.
- Corrosion Inhibitors: Specific secondary amines are employed as corrosion inhibitors in various industrial settings.
- Solvents: Some secondary amines are used as solvents in organic synthesis.
Importance of Understanding Secondary Amines in Different Fields
The role of secondary amines extends beyond their simple chemical structure. Understanding their behavior is critical in various fields:
- Medicinal Chemistry: Designing drugs with specific properties necessitates understanding how secondary amines interact with biological targets. Their basicity, hydrogen bonding capability, and steric hindrance influence drug efficacy and bioavailability.
- Materials Science: The unique properties of secondary amines allow for the synthesis of materials with desired characteristics, such as polymers with enhanced flexibility, strength, or reactivity. Their reactivity in crosslinking reactions enables the creation of advanced materials.
- Environmental Chemistry: The presence and reactivity of secondary amines in the environment can have significant consequences, influencing the fate of pollutants and their impact on ecosystems. Understanding their degradation pathways and interactions with other chemicals is essential for environmental monitoring and remediation efforts.
Conclusion: Beyond the General Representation
The general representation R₂NH, while providing a foundational understanding, only hints at the rich diversity and multifaceted nature of secondary amines. Their specific properties and reactivity are highly dependent on the nature of the R groups. The careful consideration of these substituents' electronic and steric effects is crucial for predicting and manipulating the chemical behavior of secondary amines in diverse applications. From the pharmaceutical industry to materials science and environmental studies, a deep understanding of these compounds is paramount for innovation and responsible application. Further exploration into specific examples and reactions will significantly enhance one's grasp of this important class of organic compounds. This detailed examination hopefully sheds light on the complex world beyond the simple R₂NH representation.
Latest Posts
Latest Posts
-
How Tall Is 82 Inches In Feet
Mar 31, 2025
-
How Long Should It Take To Boil Water
Mar 31, 2025
-
How Many Inches Is 4 Ft 9
Mar 31, 2025
-
Words That End In C K
Mar 31, 2025
-
101 Do F Bang Bao Nhieu Do C
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Which Is A General Representation Of A Secondary Amine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
