Which Is Classified As An Inner Transition Element
Kalali
Mar 15, 2025 · 6 min read
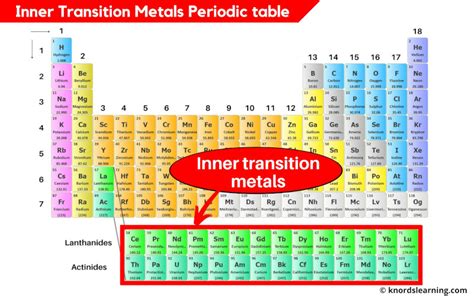
Table of Contents
Which Elements are Classified as Inner Transition Elements? A Deep Dive into the f-block
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring properties. While most are familiar with the s-block (alkali and alkaline earth metals) and p-block (containing diverse elements like halogens and noble gases), the inner transition elements, often relegated to a separate section at the bottom, hold a unique position and fascinating properties. This article delves into the characteristics that classify elements as inner transition elements, exploring their electronic configurations, chemical behavior, and notable applications.
Understanding Electron Configuration: The Key to Classification
The defining characteristic of inner transition elements lies in their electron configuration. Unlike elements in the s, p, and d blocks, where electrons fill the outermost s, p, and d orbitals respectively, inner transition elements see electrons filling the f orbitals. This is why they're also known as the f-block elements.
The Filling of the f-orbitals: A Gradual Process
The f orbitals, with their complex shapes and higher energy levels, are filled after the (n-2)f orbitals. This means that the filling of the 4f orbitals begins after the 6s and 5d orbitals are partially or fully occupied. This delayed filling is what leads to their placement beneath the main body of the periodic table. The gradual filling of these f orbitals significantly influences their chemical and physical properties.
Lanthanides: Filling the 4f orbitals
The first row of inner transition elements comprises the lanthanides, also known as the rare earth elements. These elements, from cerium (Ce) to lutetium (Lu), exhibit a gradual filling of the 4f orbitals. Their similar chemical properties make separation and purification challenging, a significant aspect of their study and application.
Key characteristics of Lanthanides:
- Similar Chemical Properties: The subtle differences in their 4f electron configurations lead to surprisingly similar chemical behavior. This makes their separation a complex chemical engineering challenge.
- Variable Oxidation States: Many lanthanides exhibit multiple oxidation states, primarily +3, but also +2 and +4 in some cases. This variability adds complexity to their chemical interactions.
- Magnetic Properties: Several lanthanides exhibit strong paramagnetism due to unpaired electrons in their 4f orbitals. This property is exploited in various applications.
- Catalytic Properties: Some lanthanides and their compounds are effective catalysts in various chemical reactions, particularly in petroleum refining and organic synthesis.
Actinides: Filling the 5f orbitals
The second row of inner transition elements consists of the actinides, ranging from thorium (Th) to lawrencium (Lr). Similar to the lanthanides, the actinides are characterized by the filling of the 5f orbitals. However, the actinides are notably different due to their radioactivity.
Key characteristics of Actinides:
- Radioactivity: All actinides are radioactive, with varying degrees of instability. This radioactivity poses both challenges and opportunities in their study and application.
- Variable Oxidation States: Actinides also display variable oxidation states, often higher than those seen in lanthanides, influencing their reactivity and chemical behavior.
- Nuclear Properties: Their nuclear properties, especially their fissile or fertile nature, are crucial in nuclear technology and energy production.
- Chemical Reactivity: Some actinides are extremely reactive, readily reacting with oxygen, halogens, and other elements. This reactivity necessitates special handling procedures.
Chemical and Physical Properties: Similarities and Differences
While both lanthanides and actinides are classified as inner transition elements, their properties exhibit some differences due to the differing energy levels of the f orbitals and the influence of relativity on the heavier actinides.
Similarities:
- Metallic Character: Both lanthanides and actinides are predominantly metallic in nature. They exhibit typical metallic properties such as high electrical and thermal conductivity.
- Large Atomic Radii: They have comparatively large atomic and ionic radii compared to elements in other blocks of the periodic table due to the poor shielding effect of the f-electrons.
- Formation of Complexes: They readily form coordination complexes with various ligands. The coordination chemistry of the lanthanides and actinides is a vast and complex area of research.
Differences:
- Radioactivity: The most significant difference is the radioactivity of actinides, absent in lanthanides (except for some artificially produced isotopes).
- Oxidation States: Actinides tend to exhibit a wider range of oxidation states than lanthanides, often showing higher oxidation states.
- Reactivity: Actinides are generally more reactive than lanthanides, exhibiting a greater tendency to form compounds.
- Electronic Configuration Irregularities: While lanthanides show a relatively regular filling of 4f orbitals, some irregularities are observed in the actinide series due to the close energy levels of the 5f and 6d orbitals.
Applications of Inner Transition Elements: A Wide Spectrum
The applications of inner transition elements are surprisingly diverse, spanning various fields from everyday materials to high-tech applications.
Lanthanides in Everyday Life and Technology:
- Lighting: Lanthanides like cerium and europium are crucial in lighting applications, notably in fluorescent lamps and high-intensity discharge lamps.
- Magnets: Neodymium magnets, incorporating neodymium (a lanthanide), are incredibly strong and are used in various applications ranging from headphones to wind turbines.
- Catalysis: Lanthanides and their oxides are efficient catalysts in various industrial processes, including petroleum cracking and the production of synthetic fuels.
- Alloys: Lanthanides improve the strength and other properties of various metals, making them vital in alloys for aerospace and other applications.
- Medical Applications: Some lanthanide compounds find use in medical imaging techniques like magnetic resonance imaging (MRI).
Actinides and Nuclear Technology:
- Nuclear Fuel: Uranium and plutonium, key actinides, are essential components of nuclear fuels used in nuclear reactors for power generation.
- Nuclear Weapons: Plutonium's fissile nature makes it a crucial component in nuclear weapons.
- Radioactive Tracers: Certain actinides are used as radioactive tracers in various scientific and medical applications, helping to study chemical reactions and biological processes.
- Radiotherapy: Certain actinides and their decay products find applications in radiotherapy to treat cancer.
Challenges and Future Research:
Despite their vast applications, the study and utilization of inner transition elements face several challenges:
- Separation and Purification: Separating lanthanides due to their similar chemical properties remains a significant challenge requiring advanced separation techniques.
- Radioactive Waste Management: The radioactive nature of actinides poses serious environmental concerns requiring safe and effective waste management strategies.
- Toxicity: Some actinides and their compounds are highly toxic, necessitating careful handling and protective measures.
- Resource Availability: Many rare earth elements are not uniformly distributed geographically, creating supply chain concerns and geopolitical issues.
Future research will likely focus on developing more efficient and sustainable methods for separating and purifying these elements, improving our understanding of their unique properties, and developing new applications in various fields such as materials science, energy, and medicine. The potential for innovation in this area remains vast.
Conclusion: Inner Transition Elements – A Unique and Crucial Part of the Periodic Table
In conclusion, inner transition elements, characterized by their filling of the f orbitals, occupy a unique and crucial position in the periodic table. Their unique chemical and physical properties, ranging from strong magnetic properties to radioactivity, lead to a vast array of applications in various sectors. Understanding their properties and addressing the challenges in their study and utilization will be vital for future advancements in technology and scientific discovery. Further research into the complex chemistry and physics of these elements will undoubtedly unveil further insights and new applications in the years to come, enriching our understanding of the world around us.
Latest Posts
Latest Posts
-
Which Would Allow Humans To Access Groundwater
Mar 15, 2025
-
What Are The Common Multiples Of 15 And 25
Mar 15, 2025
-
What Is 2 18 As A Percent
Mar 15, 2025
-
How To Find Molar Mass Of Gas
Mar 15, 2025
-
How Many Inches Is 19 Feet
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Which Is Classified As An Inner Transition Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
