Which Polymers Are Composed Of Amino Acids
Kalali
Mar 29, 2025 · 6 min read
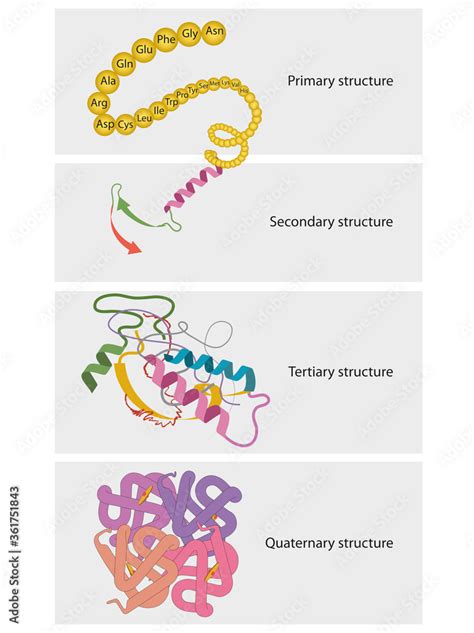
Table of Contents
- Which Polymers Are Composed Of Amino Acids
- Table of Contents
- Which Polymers are Composed of Amino Acids? Delving into the World of Proteins
- What are Amino Acids? The Building Blocks of Proteins
- The 20 Standard Amino Acids: A Diverse Group
- Peptide Bonds: Linking Amino Acids
- Protein Structure: From Primary to Quaternary
- 1. Primary Structure: The Amino Acid Sequence
- 2. Secondary Structure: Local Folding Patterns
- 3. Tertiary Structure: The 3D Arrangement
- 4. Quaternary Structure: Multiple Polypeptide Chains
- Post-Translational Modifications: Fine-Tuning Protein Function
- Beyond the 20 Standard Amino Acids: Non-Standard Amino Acids
- Proteins and Their Diverse Roles: A Biological Tapestry
- Conclusion: A Complex and Dynamic World
- Latest Posts
- Related Post
Which Polymers are Composed of Amino Acids? Delving into the World of Proteins
Proteins, the workhorses of life, are the quintessential polymers composed of amino acids. Understanding their structure, function, and the diverse roles they play is crucial in various scientific fields, from medicine and biology to materials science and engineering. This article will delve deep into the world of proteins, exploring the different types of amino acids that form them, the various levels of protein structure, and the implications of their unique properties.
What are Amino Acids? The Building Blocks of Proteins
Amino acids are the fundamental monomers that link together to form polypeptide chains, which then fold into functional proteins. Each amino acid possesses a central carbon atom (the α-carbon) bonded to four groups:
- An amino group (-NH₂): This group is basic and gives amino acids their name.
- A carboxyl group (-COOH): This group is acidic.
- A hydrogen atom (-H): A simple hydrogen atom.
- A side chain (R-group): This variable group is what differentiates the 20 standard amino acids found in proteins. The R-group's properties (size, charge, polarity, hydrophobicity) significantly influence the protein's overall structure and function.
The 20 Standard Amino Acids: A Diverse Group
The 20 standard amino acids can be broadly classified based on their side chain properties:
-
Nonpolar, aliphatic amino acids: These possess hydrophobic side chains, meaning they tend to avoid water. Examples include Glycine (Gly, G), Alanine (Ala, A), Valine (Val, V), Leucine (Leu, L), Isoleucine (Ile, I), and Methionine (Met, M).
-
Aromatic amino acids: These have ring structures in their side chains, often absorbing UV light. Examples include Phenylalanine (Phe, F), Tyrosine (Tyr, Y), and Tryptophan (Trp, W).
-
Polar, uncharged amino acids: These have side chains that can form hydrogen bonds but do not carry a net charge at physiological pH. Examples include Serine (Ser, S), Threonine (Thr, T), Cysteine (Cys, C), Asparagine (Asn, N), and Glutamine (Gln, Q).
-
Positively charged (basic) amino acids: These have side chains with a positive charge at physiological pH. Examples include Lysine (Lys, K), Arginine (Arg, R), and Histidine (His, H).
-
Negatively charged (acidic) amino acids: These have side chains with a negative charge at physiological pH. Examples include Aspartic acid (Asp, D) and Glutamic acid (Glu, E).
Peptide Bonds: Linking Amino Acids
Amino acids are linked together through peptide bonds, which are amide bonds formed between the carboxyl group of one amino acid and the amino group of another. This reaction involves the removal of a water molecule, a dehydration synthesis. A chain of amino acids linked by peptide bonds is called a polypeptide. The sequence of amino acids in a polypeptide chain is known as its primary structure. This sequence is dictated by the genetic code.
Protein Structure: From Primary to Quaternary
The structure of a protein dictates its function. There are four levels of protein structure:
1. Primary Structure: The Amino Acid Sequence
The primary structure is simply the linear sequence of amino acids in the polypeptide chain. Even a single change in this sequence (a mutation) can drastically alter the protein's function. This is crucial for understanding genetic diseases caused by mutations in protein-coding genes.
2. Secondary Structure: Local Folding Patterns
Secondary structure refers to the local folding patterns of the polypeptide chain, stabilized by hydrogen bonds between the backbone atoms. Common secondary structures include:
-
α-helices: Right-handed coiled structures stabilized by hydrogen bonds between the carbonyl oxygen of one amino acid and the amide hydrogen of an amino acid four residues down the chain.
-
β-sheets: Extended, pleated sheet-like structures formed by hydrogen bonds between carbonyl oxygens and amide hydrogens of adjacent polypeptide chains or segments of the same chain. β-sheets can be parallel (chains run in the same direction) or antiparallel (chains run in opposite directions).
-
Loops and turns: Regions connecting α-helices and β-sheets, often containing glycine and proline, which are known for their flexibility.
3. Tertiary Structure: The 3D Arrangement
Tertiary structure refers to the three-dimensional arrangement of the entire polypeptide chain. This structure is determined by a variety of interactions between the side chains (R-groups) of the amino acids:
-
Hydrophobic interactions: Nonpolar side chains cluster together in the protein's interior, away from water.
-
Hydrophilic interactions: Polar and charged side chains interact with water molecules on the protein's surface.
-
Hydrogen bonds: Formation of hydrogen bonds between various side chains.
-
Ionic bonds (salt bridges): Electrostatic attractions between oppositely charged side chains.
-
Disulfide bonds: Covalent bonds between cysteine residues, forming strong cross-links within the protein.
The tertiary structure is crucial for the protein's function as it dictates the precise arrangement of active sites, binding pockets, and other functional regions.
4. Quaternary Structure: Multiple Polypeptide Chains
Some proteins consist of multiple polypeptide chains (subunits) assembled into a larger complex. This overall arrangement is the quaternary structure. The subunits can be identical or different, and they interact through the same types of forces that stabilize tertiary structure. Examples of proteins with quaternary structure include hemoglobin (four subunits) and many enzymes.
Post-Translational Modifications: Fine-Tuning Protein Function
After synthesis, many proteins undergo post-translational modifications that further alter their structure and function. These modifications include:
- Glycosylation: Addition of carbohydrate groups.
- Phosphorylation: Addition of phosphate groups.
- Acetylation: Addition of acetyl groups.
- Ubiquitination: Addition of ubiquitin molecules.
These modifications can affect protein stability, activity, localization, and interactions with other molecules.
Beyond the 20 Standard Amino Acids: Non-Standard Amino Acids
While 20 amino acids are considered standard, other amino acids can be incorporated into proteins through various mechanisms. These non-standard amino acids often play specialized roles in protein structure or function, often arising from post-translational modifications. Examples include hydroxyproline (found in collagen), selenocysteine (containing selenium), and phosphoserine. These modifications expand the functional diversity of proteins beyond what's achievable with the standard 20.
Proteins and Their Diverse Roles: A Biological Tapestry
Proteins perform an astonishing array of functions essential for life:
- Enzymes: Catalyze biochemical reactions.
- Structural proteins: Provide support and shape to cells and tissues (e.g., collagen, keratin).
- Transport proteins: Carry molecules across cell membranes or throughout the body (e.g., hemoglobin).
- Motor proteins: Generate movement (e.g., myosin, kinesin).
- Hormones: Chemical messengers that regulate physiological processes (e.g., insulin, glucagon).
- Antibodies: Defend the body against pathogens.
- Receptors: Receive and transmit signals from the environment.
- Storage proteins: Store essential nutrients (e.g., ferritin).
The diverse roles of proteins stem from their incredible structural versatility, which is ultimately determined by the sequence and interactions of their constituent amino acids.
Conclusion: A Complex and Dynamic World
The polymers composed of amino acids – proteins – are truly remarkable molecules. Their intricate structures, diverse functions, and dynamic interactions underpin virtually all aspects of life. Understanding the properties of individual amino acids, the principles of protein folding, and the various post-translational modifications is crucial for comprehending biological processes at a molecular level. Further research continues to unravel the complexities of the protein world, revealing new insights into their roles in health, disease, and biotechnology. The study of proteins remains a vibrant and essential field, promising advancements in medicine, materials science, and our fundamental understanding of life itself.
Latest Posts
Related Post
Thank you for visiting our website which covers about Which Polymers Are Composed Of Amino Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
