Which Substance Can Be Decomposed By Chemical Means
Kalali
Mar 17, 2025 · 6 min read
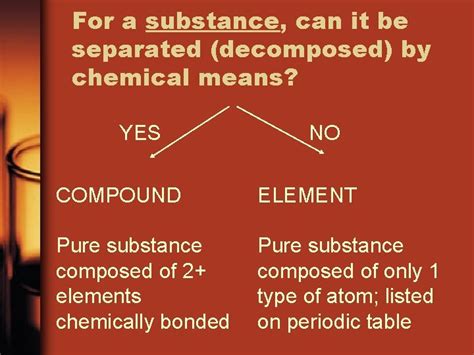
Table of Contents
Which Substances Can Be Decomposed by Chemical Means?
Decomposition reactions, a cornerstone of chemistry, involve the breakdown of a single compound into two or more simpler substances. Understanding which substances are susceptible to this type of reaction is crucial in various fields, from industrial processes to environmental science. This article delves deep into the world of chemical decomposition, exploring the types of substances that readily undergo this transformation and the factors influencing their decomposition.
Understanding Chemical Decomposition
Chemical decomposition, also known as analysis, is a type of chemical reaction where a single compound breaks down into two or more simpler substances. This process is fundamentally different from physical changes, such as melting or boiling, where the chemical composition of the substance remains unaltered. In chemical decomposition, the original substance's chemical bonds are broken, forming new substances with distinct properties. This breakdown usually requires an input of energy, which can be in the form of heat, electricity, light, or the addition of a catalyst.
Key Characteristics of Decomposition Reactions:
- Single reactant: Decomposition reactions start with only one reactant, a compound.
- Multiple products: The reaction yields two or more simpler products, which can be elements or other compounds.
- Energy input: Typically, energy is required to initiate and sustain the decomposition process.
- Irreversible (usually): While some decomposition reactions can be reversed under specific conditions, many are irreversible under normal circumstances.
Types of Substances Susceptible to Chemical Decomposition
A wide variety of substances can be decomposed chemically, but the ease of decomposition and the resulting products vary significantly depending on the substance's chemical structure and the conditions applied. Let's explore some key categories:
1. Metal Carbonates:
Metal carbonates, compounds containing a metal cation and the carbonate anion (CO₃²⁻), are readily decomposed by heat. This thermal decomposition typically yields a metal oxide and carbon dioxide gas.
Example: Calcium carbonate (CaCO₃), a major component of limestone, decomposes upon heating:
CaCO₃(s) → CaO(s) + CO₂(g)
This reaction is widely used in the production of quicklime (CaO), an important industrial material. Other metal carbonates, such as magnesium carbonate and zinc carbonate, exhibit similar behavior. The ease of decomposition often depends on the metal's reactivity; more reactive metals tend to form more stable carbonates, requiring higher temperatures for decomposition.
2. Metal Hydroxides:
Metal hydroxides, containing a metal cation and the hydroxide anion (OH⁻), also undergo thermal decomposition, generally producing a metal oxide and water.
Example: Copper(II) hydroxide (Cu(OH)₂) decomposes upon heating:
Cu(OH)₂(s) → CuO(s) + H₂O(g)
The decomposition temperature varies depending on the metal involved. Some metal hydroxides are relatively stable and require high temperatures for decomposition, while others decompose at relatively lower temperatures.
3. Metal Chlorates:
Metal chlorates, containing a metal cation and the chlorate anion (ClO₃⁻), decompose upon heating to yield a metal chloride and oxygen gas. This reaction is often vigorous and can be exothermic.
Example: Potassium chlorate (KClO₃) decomposes upon heating:
2KClO₃(s) → 2KCl(s) + 3O₂(g)
This decomposition is crucial in the laboratory preparation of oxygen gas. The reaction is often catalyzed by manganese(IV) oxide (MnO₂), which lowers the activation energy required for decomposition. Caution must be exercised when performing this experiment due to the potential for vigorous oxygen evolution.
4. Metal Oxides:
Certain metal oxides, especially those of less reactive metals, can be decomposed under specific conditions. For instance, some metal oxides can be reduced by heating with carbon or hydrogen, yielding the free metal and carbon dioxide or water, respectively.
Example: The reduction of copper(II) oxide (CuO) with hydrogen:
CuO(s) + H₂(g) → Cu(s) + H₂O(g)
5. Organic Compounds:
A vast array of organic compounds, which are carbon-based compounds, can undergo decomposition through various mechanisms. Thermal decomposition, often called pyrolysis, is a common method. This involves heating the organic compound in the absence of oxygen, leading to the formation of simpler organic molecules and sometimes carbon.
Example: The pyrolysis of wood produces charcoal, a form of carbon, along with various volatile organic compounds. The specific products depend strongly on the structure of the starting organic compound and the pyrolysis conditions.
Other decomposition mechanisms for organic compounds include:
- Hydrolysis: Decomposition using water. Esters, for instance, undergo hydrolysis to form carboxylic acids and alcohols.
- Oxidation: Decomposition using oxygen. This process is often slow and requires a catalyst or high temperatures. Complete oxidation often yields carbon dioxide and water.
- Photodecomposition: Decomposition initiated by light. Many organic compounds, especially those containing chromophores, are susceptible to photodecomposition, breaking down under exposure to UV light.
6. Hydrogen Peroxide:
Hydrogen peroxide (H₂O₂) is a relatively unstable compound that decomposes readily into water and oxygen gas. This decomposition can be catalyzed by various substances, including manganese(IV) oxide and iodide ions.
2H₂O₂(aq) → 2H₂O(l) + O₂(g)
This decomposition reaction is exothermic, releasing heat.
7. Ammonium Salts:
Ammonium salts, which contain the ammonium cation (NH₄⁺), often decompose upon heating, releasing ammonia gas (NH₃). The other products depend on the specific anion present in the salt.
Example: Ammonium chloride (NH₄Cl) decomposes upon heating:
NH₄Cl(s) → NH₃(g) + HCl(g)
8. Hydrates:
Hydrates are compounds containing water molecules incorporated into their crystal structure. Many hydrates lose their water molecules upon heating, a process called dehydration.
Example: Copper(II) sulfate pentahydrate (CuSO₄·5H₂O) loses its water molecules upon heating:
CuSO₄·5H₂O(s) → CuSO₄(s) + 5H₂O(g)
Factors Affecting Chemical Decomposition
Several factors influence the rate and extent of chemical decomposition:
- Temperature: Increased temperature generally accelerates decomposition reactions by providing the necessary activation energy to break chemical bonds.
- Pressure: Pressure can play a role in certain decomposition reactions, particularly those involving gases.
- Catalyst: Catalysts can significantly lower the activation energy required for decomposition, increasing the reaction rate.
- Concentration: The concentration of the reactant can affect the rate of decomposition, particularly in solution-phase reactions.
- Surface area: For solid reactants, a larger surface area generally leads to faster decomposition.
Applications of Chemical Decomposition
Chemical decomposition finds widespread applications in various fields:
- Industrial production: The production of metals from their ores often involves decomposition reactions, as does the manufacture of various chemicals and materials.
- Environmental science: Decomposition processes play a vital role in the natural breakdown of organic matter and the cycling of nutrients.
- Analytical chemistry: Decomposition reactions are used in analytical techniques to determine the composition of unknown substances.
- Medicine: Certain medications utilize decomposition reactions as part of their therapeutic effect.
Conclusion
Chemical decomposition is a fundamental chemical process with broad implications across numerous disciplines. A vast array of substances, from simple inorganic compounds to complex organic molecules, are susceptible to decomposition under appropriate conditions. Understanding the factors that influence decomposition and the resulting products is crucial for controlling and harnessing this powerful chemical transformation for various practical applications. Further research continues to reveal the intricate details of decomposition reactions, leading to new discoveries and innovations in various fields. The information provided here serves as a foundational understanding of this vital area of chemistry. Always remember to prioritize safety when working with chemical reactions.
Latest Posts
Latest Posts
-
What Is 1 5 8 As A Decimal
Mar 17, 2025
-
3 Feet 6 Inches In Cm
Mar 17, 2025
-
What Is 26 Out Of 30 As A Percentage
Mar 17, 2025
-
How Many Feet Is 26 In
Mar 17, 2025
-
What Is 6 Out Of 20 As A Percentage
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Which Substance Can Be Decomposed By Chemical Means . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
